Introduction
The prognosis of primary central nervous system lymphoma (PCNSL) has improved with the introduction of high-dose methotrexate (HD-MTX)-based chemotherapeutic regimens. However, the optimal consolidation therapy is still uncertain. From the early 2000s, to avoid the late CNS complications of whole-brain radiation therapy (WBRT), we have been treating PCNSL with upfront high-dose chemotherapy, named LEED (melphalan: L-PAM, cyclophosphamide: CPA, etoposide: ETP, and dexamethasone: DEX), followed by autologous peripheral blood stem cell transplantation (ASCT). In this study, we aimed to explore the efficacy of ASCT following LEED against PCNSL and its safety mainly for CNS.
Methods
The outcomes of patients who were diagnosed with PCNSL, who were younger than 75 years of age at the time of diagnosis, and who could receive at least 1 cycle of treatment were obtained from clinical records from Jan 2003 to Dec 2018 at our hospital. HIV-positive patients and those with extra-CNS lesions were excluded from data collection. The treatment results and adverse events were retrospectively analyzed. Our basic treatment policy is as follows. We administer 3 cycles of HD-MTX-based regimens. Then, 2 cycles of high-dose (2 g/m2) Cytarabine (AraC) (HD-AraC) are administered. Peripheral blood stem cell harvest is planned during the 2nd course of HD-AraC. LEED is adopted as a conditioning regimen for ASCT, which consists of CPA (60 mg/kg from day -4 to -3), ETP (250 mg/m2 every 12 h from day -4 to -2), L-PAM (130 mg/m2 on day -1), and DEX (48 mg/day from day -4 to -1). Rituximab is administered during each course of HD-MTX and HD-AraC only if CD20 is histologically detected in the specimen. WBRT is sometimes added for patients who could not achieve complete remission (CR) with or without ASCT if they keep fit at that time and were thought to be tolerable. We divided patients into ASCT and non-ASCT group for comparison.
Results
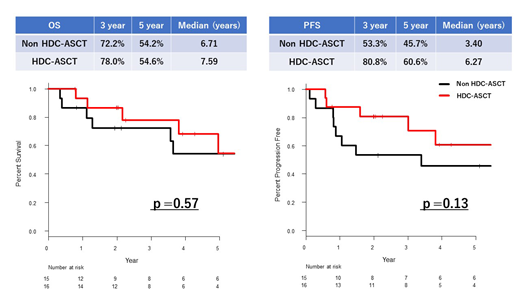
The median follow-up period was 43 months (4-172) for 31 patients. Sixteen patients received ASCT. The median age of patients who received ASCT was 58 years (35-69), and that of non-ASCT patients was 60 years (33-74). ECOG PS, LD, sIL2R, and prognostic score of PCNSL at diagnosis showed no significant differences between the 2 groups. All patients who received ASCT had already shown treatment response greater than partial remission (PR) before ASCT (CR=6, PR=10); non-ASCT group had acquired similar response after HD-AraC (CR=10, PR=2). Three patients in the ASCT group and 6 patients in the non-ASCT group received WBRT. Overall survival rate (OS) at 3 years from the beginning of initial treatment and median OS were almost the same in both groups (3 year-OS: ASCT vs. Non-ASCT: 78.0% vs. 72.2%, median OS: 7.6 years vs. 6.7 years, P=0.57). The progression free survival rate (PFS) at 3 years was 80.8% in the ASCT group and 53.3% in the non-ASCT group (median PFS: 6.3 years vs. 3.4 years, P=0.13). The overall response rate, which was defined as proportion of the patients whose best response after ASCT was greater than PR, in the ASCT group was 94% (CR=13, PR=2). The 5-year relapse rate (RR) in the ASCT group was 29.3%, and that in the non-ASCT group was 47.6% (P=0.21). Although grade 3-4 adverse events occurred in all patients (febrile neutropenia 88%, diarrhea 19%) during the course of ASCT, all of them achieved neutrophil engraftment. One patient developed MDS 4 years after ASCT, which was fatal. Long-term follow-up revealed that no patient in the ASCT group experienced adverse neurological events; one patient in the non-ASCT group, who received WBRT, developed leukoencephalopathy. Fifteen patients were not able to proceed to ASCT, mainly because of harvest failure (27%), low performance status (27%), and comorbidities (20%).
Conclusions
Our study suggested the safety and efficacy of ASCT following LEED against PCNSL. PFS and RR in the ASCT group tended to be better than that in the non-ASCT group even though the study scale would not be enough to show statistical significance. Leukoencephalopathy, one of the biggest concerns associated with WBRT, was not experienced in the ASCT group. In summary, we developed the strategy aiming for upfront ASCT against PCNSL. This strategy proved to be promising in terms of the safety and efficacy. However, since this retrospective study analyzed a limited number of patients, further investigation is essential to establish the superiority of ASCT over WBRT.
Yokota:Celgene: Honoraria; Bristol-Myers Squibb: Honoraria; Novartis: Honoraria; Ono: Honoraria; Takeda: Honoraria. Miyao:Celgene Corporation: Honoraria; Novartis: Honoraria; Bristol-Myers Squibb: Honoraria. Takeuchi:Novartis: Honoraria; Eisai: Honoraria; Celgene: Honoraria; Takeda: Honoraria; Ono: Honoraria. Kuwano:Bristol-Myers Squibb: Honoraria; Celgene: Honoraria; Takeda: Honoraria. Inagaki:Mundipharma: Honoraria; Eisai: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Honoraria. Sawa:Mundi Pharma: Honoraria; Otsuka Pharmaceutical: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria; Bristol-Myers Squibb: Honoraria; Takeda: Honoraria; Sumitomo Dainippon Pharma: Honoraria; Asahi-Kasei: Honoraria; Kyowa-Hakko Kirin: Honoraria; Pfizer Japan Inc.: Honoraria; Eisai: Honoraria; Shire: Honoraria; Nippon Shinyaku: Honoraria; Astellas Pharma Inc.: Honoraria; Ono Pharmaceutical Co., Ltd: Honoraria; MSD: Honoraria; Novartis: Honoraria; Sanofi: Honoraria; Celgene: Honoraria; Mochida: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal