Introduction
CD19 directed CAR T cells have shown potent activity in relapsed/refractory (R/R) aggressive B-cell lymphomas (B-NHL) leading to the FDA approval of axicabtagene ciloleucel (axi-cel, Oct 2017) and tisagenlecleucel (tisa-cel, May 2018). Initial reports on commercial application of axi-cel suggest many patients (pts) would not have met eligibility criteria for the ZUMA-1 clinical trial, yet outcomes and toxicities appeared similar (Nastoupil LJ, et al. Blood 2018 132:91 and Jacobson CA, et al. Blood 2018 132:92). No data on the "real world" application of tisa-cel is available. We performed a multicenter retrospective study to include both approved commercial products, axi-cel and tisa-cel, given in centers that had the option of prescribing either product. We evaluate patterns of use, efficacy, and safety.
Methods
We retrospectively analyzed data from pts who underwent apheresis for commercial axi-cel or tisa-cel from 8 US academic centers. Data collection started after 5/1/2018, following FDA approval of tisa-cel when centers would have a choice to prescribe either axi-cel or tisa-cel for B-NHL. Centers were invited to participate if they were certified to administer both products. Patient and treatment characteristics were summarized descriptively. Response and toxicity were reported with 95% exact binomial CIs.
Results
As of 7/31/2019, 242 pts underwent apheresis for commercial CAR T-cell products. Of these, 163 (67%) underwent apheresis for axi-cel, and 79 (33%) for tisa-cel. 14 (9%) axi-cel and 3 (4%) tisa-cel pts died prior to CAR T-cell infusion from lymphoma progression, and 1 (1%) tisa-cel pt was not infused for other reasons.
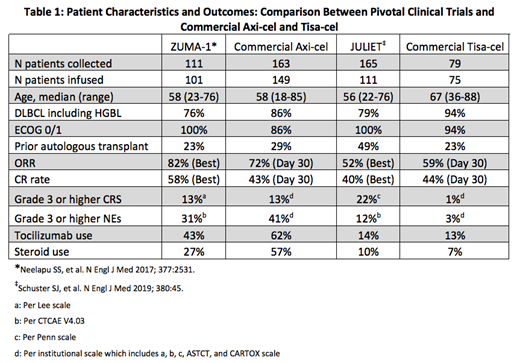
Detailed baseline pt characteristics were available for 180/242 pts (Table 1). Median age at apheresis was 58 years (range: 18-85) for axi-cel pts and 67 years (range: 36-88) for tisa-cel pts. ECOG PS was 0-1 in 86% of axi-cel and 94% of tisa-cel pts. By histology, 77% of axi-cel pts had DLBCL, 13% TFL, 9% HGBL and 2% PMBCL. Similarly, 81% of tisa-cel pts had DLBCL, 13% HGBL, and 6% TFL. The median number of prior therapies was 3 (range: 2-11) for axi-cel and 4 (range: 2-9) for tisa-cel pts. Prior autologous stem cell transplant was performed in 29% of axi-cel and 23% of tisa-cel pts, respectively. Bridging therapy was given in 61% of axi-cel and 72% of tisa-cel pts. Median time from apheresis to CAR T-cell infusion was 28 days for axi-cel and 44 days for tisa-cel. CAR T-cell infusion was inpatient in 100% of axi-cel and 39% of tisa-cel pts.
Safety was evaluable in 213 pts. CRS was graded according to institutional practices (CARTOX (38%), Penn scale (31%), ASTCT (19%), and Lee scale (11%)). NEs were graded per CARTOX (80%), ASTCT (19%), or CTCAE V4.03 (1%). Grade ≥3 CRS and NEs occurred in 13% and 41% of axi-cel pts and 1% and 3% of tisa-cel pts. The median onset of CRS and NEs was 2 and 6 days in axi-cel, and 3 and 5 days in tisa-cel treated pts, respectively. Tocilizumab was administered in 62% of axi-cel pts with 57% receiving steroids. In tisa-cel pts, tocilizumab was administered in 13% of cases, with 7% receiving steroids. 12 deaths (8%) unrelated to lymphoma progression occurred in axi-cel pts at a median of 57 days (range: 6-373) with 5 due to infectious complications, 4 due to grade 5 NEs, 1 due to cardiac disease, 1 due to pulmonary hemorrhage, and 1 due to HLH. 4 deaths (6%) unrelated to lymphoma progression occurred in tisa-cel pts at a median of 48 days (range: 25-146) with 2 due to infectious complications, 1 due to cardiac disease, and 1 due to unknown causes.
Response assessment was performed for infused pts at day 30 and/or day 90, or in those determined to have clinical progression. Of 120 axi-cel pts evaluable at day 30, the ORR was 72% with 43% achieving a CR. Of the 32 tisa-cel pts evaluable at day 30, the ORR was 59% with 44% achieving a CR. At day 90, the ORR for axi-cel was 52% with 39% achieving a CR, while for tisa-cel the ORR was 48% with 39% achieving a CR.
Conclusions
Efficacy outcomes in the commercial setting appear similar to responses seen in the pivotal clinical trials. Though different toxicity grading scales were employed, tisa-cel appears to be associated with less CRS and NEs. Data from a larger group of pts treated at additional centers are being gathered. Analyses of usage patterns and updated outcomes with uniform ASTCT toxicity grading will be presented in an effort to better understand therapeutic decision making.
Riedell:Novartis: Research Funding; Verastem: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Bayer: Honoraria, Speakers Bureau. Nastoupil:Spectrum: Honoraria; TG Therapeutics: Honoraria, Research Funding; Novartis: Honoraria; Janssen: Honoraria, Research Funding; Gilead: Honoraria; Genentech, Inc.: Honoraria, Research Funding; Bayer: Honoraria; Celgene: Honoraria, Research Funding. Maziarz:Kite: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Incyte: Consultancy, Honoraria; Celgene/Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. McGuirk:Gamida Cell: Research Funding; Pluristem Ltd: Research Funding; Novartis: Research Funding; Fresenius Biotech: Research Funding; Astellas: Research Funding; Bellicum Pharmaceuticals: Research Funding; Kite Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Juno Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; ArticulateScience LLC: Other: Assistance with manuscript preparation. Oluwole:Pfizer: Consultancy; Spectrum: Consultancy; Gilead Sciences: Consultancy; Bayer: Consultancy. Bachanova:Novartis: Research Funding; Celgene: Research Funding; Kite: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Gamida Cell: Research Funding; GT Biopharma: Research Funding. Hwang:Tmunity: Research Funding; Novartis: Research Funding. Schuster:Pharmacyclics: Honoraria, Research Funding; Acerta: Honoraria, Research Funding; AstraZeneca: Honoraria; Loxo Oncology: Honoraria; Nordic Nanovector: Honoraria; Pfizer: Honoraria; Novartis: Honoraria, Patents & Royalties: Combination Therapies of CAR and PD-1 Inhibitors with royalties paid to Novartis, Research Funding; Celgene: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Merck: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Gilead: Honoraria, Research Funding. Perales:Bellicum: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; NexImmune: Membership on an entity's Board of Directors or advisory committees; MolMed: Membership on an entity's Board of Directors or advisory committees; Omeros: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Meyers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Nektar Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria; Medigene: Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Kyte/Gilead: Research Funding; Miltenyi: Research Funding. Bishop:Juno: Consultancy, Membership on an entity's Board of Directors or advisory committees; CRISPR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kite: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Porter:American Board of Internal Medicine: Membership on an entity's Board of Directors or advisory committees; Immunovative: Membership on an entity's Board of Directors or advisory committees; Genentech: Employment; Wiley and Sons: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding; Kite: Membership on an entity's Board of Directors or advisory committees; Glenmark Pharm: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal