Background:Zanubrutinib is an investigational, next-generation BTK inhibitor, designed to maximize BTK occupancy and minimize off-target inhibition of TEC- and EGFR-family kinases. It has been shown to be highly potent, selective, bioavailable, and irreversible with potentially advantageous pharmacokinetic and pharmacodynamic properties (Tam Blood 2019). Tislelizumab is an investigational humanized IgG4 variant monoclonal antibody with high affinity and specificity for the programmed cell death-1 (PD-1) receptor, and was engineered to minimize binding to Fc-γ receptor on macrophages in order to mitigate macrophage-driven killing of effector T-cells, which may compromise the anti-tumor activity of PD-1 inhibitors (Friedlander ASCO 2017). Zanubrutinib and tislelizumab have shown encouraging efficacy as monotherapies in phase 2 studies in patients (pts) with hematologic cancers. PD-1/PD-L1 and B-cell receptor pathway inhibitors are being evaluated in combination for various B-cell malignancies, with the expectation of an additive or synergistic effect. Updated safety and preliminary efficacy data from a phase 1b trial are presented here.
Methods:Multicenter, phase 1b trial to evaluate the safety, tolerability, and preliminary efficacy of zanubrutinib + tislelizumab. Standard 3+3 design with expanded cohorts dosed at the recommended phase 2 dose (zanubrutinib 160 mg po bid + tislelizumab 200 mg IV Q3W). Pts with locally diagnosed relapsed/refractory (R/R) aggressive non-Hodgkin lymphomas (NHL), specifically diffuse large B-cell lymphoma (DLBCL), transformed follicular lymphoma (tFL), Richter transformation (RT), and primary and secondary central nervous system lymphomas (CNSL) are included in dose expansion. Results from dose escalation, including in indolent lymphomas, were previously reported (Trotman ASH 2017).
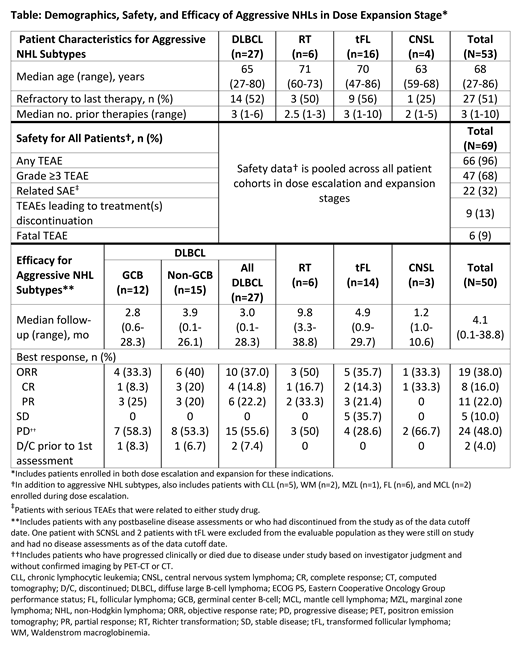
Results:As of April 10, 2019, 69 pts with B-cell malignancies were enrolled including 53 with select aggressive NHL subtypes: DLBCL (n=27), tFL (n=16), RT (n=6), and CNSL (n=4). Key patient characteristics, safety and efficacy are shown in the Table. In dose escalation, the maximum tolerated dose was not achieved; 1 dose-limiting toxicity (DLT) of treatment-related (both drugs) grade 3 drug hypersensitivity reaction, manifesting as maculopapular rash, occurred at the RP2D in a pt with RT. The pt was re-challenged and experienced recurrence (grade 4) but recovered after discontinuing study drugs. No further DLTs were observed in the other 6 evaluable pts at the RP2D. For all dosed pts (n=69), the median treatment duration was 3.0 mo (range, <0.1-33.4). The most common treatment-emergent adverse events (AEs) were diarrhea (n=16; 23.2%), fatigue (n=14; 20.3%), cough, nausea, and upper respiratory tract infection (each n=13; 18.8%). Frequent grade ≥3 AEs included neutropenia (n=9; 13%), anemia (n=6; 8.7%), and thrombocytopenia (n=4; 5.8%). Related serious AEs in ≥2 pts were immune-related (IR) enterocolitis (grade 3, n=3), anemia (grade 3, n=1; grade 4, n=1), hemolytic transfusion reaction and pneumonitis (each grade 3, n=2). Six pts (8.7%) experienced fatal AEs of multiple organ dysfunction, septic shock, abdominal pain (pt had concurrent progressive disease), sepsis, respiratory failure, and toxic epidermal necrolysis (TEN). TEN was the only fatal AE attributed to study drug (both) as the pt died despite treatment stoppage and use of antibiotics, IVIG, and corticosteroids. No serious hemorrhages have been reported to date. Atrial flutter was reported in 2 pts (1 grade 3 and serious AE). Nine pts (13.0%) discontinued ≥1 study drug due to AEs as the primary cause, which were mostly serious and immune-mediated, including pneumonitis (2 pts), IR-hepatitis, IR-enterocolitis, and IR-encephalitis (1 pt each). The preliminary objective response rate [ORR] (Cheson J Clin Oncol 2014) in evaluable pts with aggressive R/R NHLs (n=50) is 38% with a median follow-up of 4.1 mo. In non-germinal center DLBCL and RT subtypes, the ORR is 40% (6/15 pts) and 50% (3/6 pts), respectively (Table).
Conclusions: The combination of zanubrutinib and tislelizumab has shown a generally manageable toxicity profile in B-cell malignancies. IR-AEs, consistent with anti-PD-1 therapy, were observed and managed with supportive care; however, treatment cessation was necessary in a proportion of pts. Activity has been observed in aggressive subtypes of NHL. Enrollment is ongoing.
Tam:Novartis: Honoraria; BeiGene: Honoraria; Roche: Honoraria; Janssen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding. Cull:Amgen: Other: Travel, accommodation ; Glycomimetics: Other: Travel, accommodation; AbbVie: Other: Travel, accommodation. Opat:Epizyme: Research Funding; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Research Funding; Novartis: Consultancy; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Mundipharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Amgen: Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria. Gregory:Gilead: Membership on an entity's Board of Directors or advisory committees; Melbourne Haematology: Consultancy, Honoraria, Other: Travel fees and conference support, Speakers Bureau; Roche: Speakers Bureau; AbbVie: Other: grant pending, Research Funding; Celgene: Other: grant pending, Research Funding; Monash University: Research Funding; Beigene: Other: Grant pending, Research Funding; Janssen: Other: grant pending, Research Funding; MSD: Other: grant pending, Research Funding. Johnston:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen, Roche: Membership on an entity's Board of Directors or advisory committees. Roncolato:St. George Hospital: Employment. Handunnetti:Gilead: Honoraria; Abbvie: Other: Travel Grant. Prince:Takeda: Consultancy, Honoraria; Celgene: Honoraria; Allergan: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Li:Guangdong Province Hospital: Employment. Shih:BeiGene: Employment, Equity Ownership. Zhang:BeiGene: Employment, Equity Ownership. Wu:BeiGene: Employment, Equity Ownership. Liu:BeiGene: Employment, Equity Ownership. Huang:BeiGene: Employment, Equity Ownership. Trotman:BeiGene: Research Funding; Janssen: Research Funding; Celgene: Research Funding; Pharmacyclics: Research Funding; Roche: Research Funding.
Zanubrutinib and tislelizumab are investigational agents and have not yet been approved in the US
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal