M.T.J. and M.D.J. contributed equally; A.G. and F.L.L. contributed equally.
Introduction
Axicabtagene ciloleucel (axi-cel) is an autologous anti-CD19 CAR T-cell therapy that is approved for the treatment of adults with relapsed or refractory large B-cell lymphoma (LBCL) who have failed at least two prior systemic lines of therapy. In the ZUMA-1 trial that led to axi-cel approval, 93% of patients developed cytokine release syndrome (CRS) (Neelapu, Locke et al. NEJM 2018). CRS is a non-antigen-specific toxicity that occurs as a result of CAR T and bystander immune cell activation. Whether lack of development of CRS after treatment with axi-cel is associated with inferior lymphoma outcomes is unknown. Here we evaluate the outcomes of patients that did not develop CRS after receiving axi-cel in a large multicenter cohort.
Methods and Results
The US Lymphoma CAR T Consortium includes seventeen US academic centers that contribute data independently of manufacturers. As of 8/31/2018, 300 patients were apheresed with intent to manufacture standard of care axi-cel for LBCL. In this study, we analyzed the modified intent-to-treat (mITT) population of 276 patients receiving CAR T infusion with a median follow up of 9 months. In this group, 25 patients (9%) did not develop CRS and 251 patients (91%) developed CRS following axi-cel infusion. CRS was graded according to Lee criteria (Lee et al. Blood 2014) or CARTOX (Neelapu SS et al. Nat Rev Clin Oncol. 2018).
At baseline, a higher proportion of patients in the no CRS group had ECOG score of 0-1 (no CRS group 100% vs. CRS group 82%, p= 0.019) and IPI score of 0-2 (no CRS group 72% vs. CRS group 46%, p = 0.019)
After CAR T cell infusion, patients who did not develop CRS had a lower chance of developing grade 3 or higher neurotoxicity (no CRS group 4% vs. CRS group 35%, p=0.001), lower rates of ICU admission (no CRS group 8% vs. CRS group 35%, p = 0.006), and shorter length of hospital stay (median 10 days for no CRS group vs 14 days for CRS group, p < 0.001). Of the 25 patients who had grade 0 CRS, 23 (92%%) also had grade 0 neurotoxicity. In univariate analysis, no CRS was associated with lower complete response (CR) rate (no CRS group 40% vs. CRS group 66%, p=0.015) but no statistically significant difference in overall response rate (ORR) (no CRS group 72% vs. CRS group 84%, p= 0.158). In relation to CRS there was no difference in treatment related mortality among the two groups (CRS group 4% vs. no CRS group 4.4%, p = 0.85).
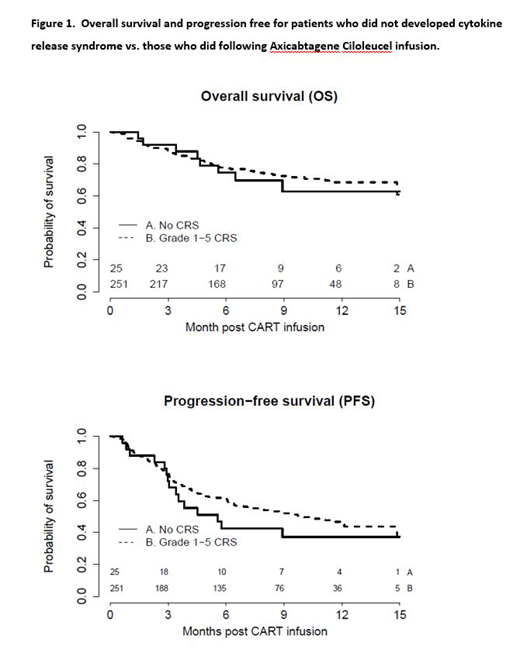
In multivariate analysis correcting for confounding features, no CRS was associated with statistically significant lower complete response rate (p = 0.002), but there was no significant difference in ORR (p = 0.13), overall survival (P=0.15), progression free survival (P=0.16), and time to progression (P= 0.14) between the two groups (figure 1.).
Conclusions
In this large cohort of LBCL patients receiving axi-cel with median follow up of 9 months, patients that did not develop CRS, compared with those that developed CRS, achieved lower rates of complete response but there was no difference in overall response rate, progression free survival, time to progression, overall survival, and treatment related mortality between the two groups.
Jain:Kite/Gilead: Consultancy. Nastoupil:Bayer: Honoraria; TG Therapeutics: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Genentech, Inc.: Honoraria, Research Funding; Gilead: Honoraria; Janssen: Honoraria, Research Funding; Novartis: Honoraria; Spectrum: Honoraria. Lunning:Spectrum: Consultancy; Seattle Genetics: Consultancy; Portola: Consultancy; OncLive: Consultancy; Novartis: Consultancy; Kite: Consultancy; Gilead Sciences, Inc.: Consultancy; DAVA: Consultancy; Bayer: Consultancy; AbbVie: Consultancy; TG Therapeutics: Consultancy, Research Funding; MiRagen: Research Funding; Juno Therapeutics: Consultancy, Research Funding; Janssen Scientific Affairs, LLC: Consultancy, Research Funding; Curis: Research Funding; VANIUM: Consultancy; Verastem: Consultancy. Reagan:Kite, A Gilead Company: Consultancy; Curis: Consultancy; Seattle Genetics: Research Funding. Oluwole:Pfizer: Consultancy; Spectrum: Consultancy; Gilead Sciences: Consultancy; Bayer: Consultancy. McGuirk:Novartis: Research Funding; Kite Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gamida Cell: Research Funding; Pluristem Ltd: Research Funding; ArticulateScience LLC: Other: Assistance with manuscript preparation; Juno Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bellicum Pharmaceuticals: Research Funding; Fresenius Biotech: Research Funding; Astellas: Research Funding. Deol:Agios: Other: Advisory board; Novartis: Other: Advisory board; Kite: Other: Advisory board. Sehgal:Juno/Celgene: Research Funding; Merck: Research Funding; Kite/Gilead: Research Funding. Goy:Acerta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; University of Nebraska: Research Funding; Astrazenca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Hakensackumc: Research Funding; Hackensack University Medical Center, RCCA: Employment; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work; Takeda: Other: Grants outside of the submitted work; COTA: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Other: leadership role for profit healthcare company; Pharmacyclics/Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Grants outside of the submitted work, Research Funding; Genentech: Other: Grants outside of the submitted work, Research Funding. Hill:Kite: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Takeda: Research Funding; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celegene: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Research Funding; TG therapeutics: Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Research Funding. Andreadis:Juno: Research Funding; Celgene: Research Funding; Novartis: Research Funding; Roche: Equity Ownership; Jazz Pharmaceuticals: Consultancy; Kite: Consultancy; Gilead: Consultancy; Genentech: Consultancy, Employment; Pharmacyclics: Research Funding; Merck: Research Funding. Munoz:Incyte: Research Funding; Portola: Research Funding; AstraZeneca: Speakers Bureau; Fosunkite: Speakers Bureau; Pfizer: Consultancy; Alexion: Consultancy; Bristol-Myers Squibb: Consultancy; Pharmacyclics /Janssen: Consultancy, Research Funding, Speakers Bureau; Bayer: Consultancy, Speakers Bureau; Merck: Consultancy; Kyowa: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene/Juno: Consultancy, Research Funding; Genentech: Consultancy, Research Funding, Speakers Bureau; Kite/Gilead: Consultancy, Research Funding, Speakers Bureau. Chavez:Janssen Pharmaceuticals, Inc.: Speakers Bureau; Genentech: Speakers Bureau; Kite Pharmaceuticals, Inc.: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Bennani:Purdue Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Purdue Pharma: Other: Advisory board; Seattle Genetics: Other: Advisory board; Seattle Genetics: Other: Advisory board; Adicet Bio: Other: Advisory board; Adicet Bio: Other: Advisory board; Adicet Bio: Other: Advisory board; Kite Pharma: Other: Advisory board; Kite Pharma: Other: Advisory board; Kite Pharma: Other: Advisory board; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Purdue Pharma: Other: Advisory board. Vose:Acerta Pharma: Honoraria, Other: Grants, Research Funding; Bristol-Meyers Squibb Company: Research Funding; Celgene Corporation: Research Funding; Incyte Corporation: Research Funding; Kite Pharma: Honoraria, Other: Grants, Research Funding; Novartis: Research Funding; Seattle Genetics: Research Funding; AbbVie: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Legend Pharmaceuticals: Honoraria. Miklos:Becton Dickinson: Research Funding; Miltenyi Biotech: Membership on an entity's Board of Directors or advisory committees; AlloGene: Membership on an entity's Board of Directors or advisory committees; Precision Bioscience: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees; Kite-Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Neelapu:Poseida: Research Funding; Cellectis: Research Funding; Precision Biosciences: Consultancy; Cell Medica: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Celgene: Consultancy, Research Funding; Allogene: Consultancy; Acerta: Research Funding; Unum Therapeutics: Consultancy, Research Funding; Pfizer: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; BMS: Research Funding; Merck: Consultancy, Research Funding; Karus: Research Funding. Ghobadi:Wugen: Consultancy; Celgene: Consultancy; EUSA: Consultancy; Kite Pharma a Gilead Company: Consultancy, Research Funding, Speakers Bureau. Locke:Novartis: Other: Scientific Advisor; Cellular BioMedicine Group Inc.: Consultancy; Kite: Other: Scientific Advisor.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal