Background: The interferons, including IFNα/IFNβ (type I) and IFNγ (type II) are essential mediators of anti-cancer immunity. To achieve efficient targeting of IFNs to tumor sites, we have developed antibody (Ab)-IFN fusion protein technology. We previously reported the antigen-specific targeting of IFNα to CD20+ target cells with efficient inhibition of proliferation, induction of apoptosis, and in vivo tumor eradiation dependent upon IFNα receptors on the tumor cell surface (Xuan et al, Blood, 2010). A fusion protein targeting human CD20 (anti-CD20-IFNα) exhibited stronger direct anti-proliferative effects, complement-dependent cytotoxicity (CDC), Ab-dependent cell-mediated cytotoxicity (ADCC), and in vivo potency against B-cell lymphoma xenograft models compared to the parent Ab rituximab (Timmerman et al, Blood 2015). Based on these results, a phase I, first-in-human, dose-escalation trial of anti-CD20-IFNα for B cell non-Hodgkin lymphoma is now underway (NCT02519270). Given the distinct properties of IFNγ from type I IFNs, including upregulation of antigen presentation, control of immune cell trafficking, and activation of T cells, NK cells, and macrophages, we hypothesized that Ab-targeted IFNγ may have anti-tumor effects mechanistically-distinct from those of Ab-IFNα fusions. We now report on the construction and characterization of anti-CD20 fusions containing IFNγ.
Methods: The VH and VL regions from antibody 2B8 recognizing human CD20 were engineered in recombinant form with mouse IgG2a constant regions, and fused at the C-terminus with mIFNγ, yielding anti-hCD20-mIFNγ. Tumor cell proliferation in vitro was measured by [3H]-thymidine incorporation, ADCC by LDH release using mouse splenocyte effectors, CDC by propidium iodide (PI) exclusion, and in vivo tumor growth assessed using the huCD20-expressing syngeneic mouse B cell lymphoma 38C13-huCD20. Tumor-infiltrating lymphocytes were measured by flow cytometry.
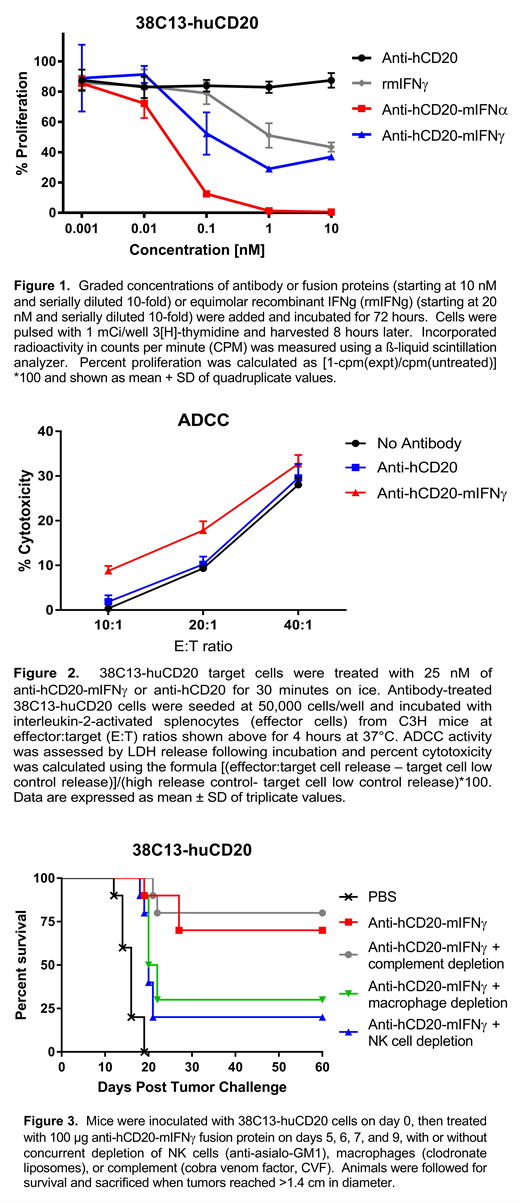
Results: Anti-hCD20-mIFNγ displayed potent IFNγ bioactivity comparable to free recombinant mIFNγ, and suppressed the in vitro proliferation of 38C13-huCD20 lymphoma cells by up to 70% (at 1 nM), though not as potently as anti-hCD20-mIFNα, which inhibited proliferation by 98% (Figure 1). Anti-hCD20-mIFNγ showed enhanced ADCC against lymphoma cells compared with the unfused, parent antibody (16-20% at E:T ratio of 20:1, versus 9-12%, respectively, p=0.0024)(Figure 2), while CDC was identical to unfused antibody. In vivo efficacy was demonstrated in mice bearing established subcutaneous 38C13-huCD20 tumors, with systemic (i.v.) injection of 100 μg anti-hCD20-mIFNγ fusion protein on days 5, 6, 7, or 5, 6, 7, 9 after tumor inoculation resulting in cure of approximately 70-80% of mice in repeated experiments. In contrast, therapy with equimolar doses of unfused, native anti-hCD20 Ab resulted in no cures. Mechanistic studies in anti-hCD20-mIFNγ fusion protein-treated mice showed that depletion of natural killer (NK) cells (using anti-asialo-GM1) significantly abrogated tumor clearance (p=0.01), while depletion of macrophages (clodronate liposomes) had lesser, borderline effects (p= 0.05)(Figure 2), and depletion of complement (cobra venom factor) or T cells (CD4+ or CD8+) had no significant effects on tumor eradication. Subcutaneous mouse B cell lymphomas treated with intratumoral injections of anti-hCD20-mIFNγ displayed increased tumor-infiltrating CD8+ T cells (mean 20.6% versus 5% in PBS-treated controls, p=0.008), and CD4+ T cells (mean 15.3% versus 6.6%).
Conclusions: Anti-hCD20-mIFNγ fusion protein has in vitro and in vivo efficacy in a syngeneic, immunocompetent model of B cell lymphoma, with NK cells and possibly macrophages implicated in the mechanism(s) of tumor eradication. Ab-targeted mIFNγ can also promote infiltration of immune cells into the tumor microenvironment. These findings may suggest a novel approach for the immunotherapy of B cell lymphomas and other cancers.
Vasuthasawat:Qwixel therapeutics LLC: Other: stake;which receives some funding through UCLA. Trinh:Qwixel therapeutics LLC: Other: stake;which receives some funding through UCLA. Morrison:Qwixel therapeutics LLC: Other: stake;which receives some funding through UCLA. Timmerman:ImmunGene: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Other: travel support, Research Funding; Merck: Research Funding; Kite, A Gilead Company: Consultancy, Honoraria, Other: travel support, Research Funding; Spectrum Pharmaceuticals: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal