Intruduction
Extranodal natural killer/T-cell lymphoma (ENKTL) is an uncommon, aggressive form of non-Hodgkin's lymphoma. Optimal therapeutic strategies have not been fully defined yet. Nasal NK/T-cell lymphomas present mostly with stage I/II disease. For stage I/II nasal lymphoma, a combination of chemotherapy and radiotherapy yields optimal results. Concomitant chemoradiotherapy and sequential chemotherapy and radiotherapy give similar response rates and survivals. For stage III/IV nasal, nonnasal, and disseminated ENKTL, systemic chemotherapy is indicated. Conventional anthracycline-based regimens are ineffective. Regimens containing L-asparaginase are most effective. Both AspaMetDex and P-Gemox is recommended as major effective combined chemotherapy regimen by NCCN guideline. Therefore, we try to evaluate the efficacy and toxicity for P-Gemox plus thalidomide and AspaMetDex followed by extensive involved field radiotherapy (EIFRT) as first-line treatment for newly diagnosed stage I/II patients and as salvage regimen for newly diagnosed stage III/IV or relapsed/refractory ENKTL in this clinical study.
Methods
We initiated a prospective, multicentre, randomized, phase II clinical trial at 12 centers in China at March 2014. Patients were randomly assigned to receive either P-Gemox+thalidomide regimen (Group A: Pegaspargase 2000U/m2; im d1, Gemcitabine 1000mg/m2; ivdrip , d1, d8. Oxaliplatin 130mg/m2; ivdrip, d1, thalidomide 100mg/d po, for one year.) or AspaMetDex regimen ( Group B: Pegaspargase 2000U/m2; im, d1, Methotrexate 3000mg/ m2; civ 6-hour, d1, calcium folinate 30mg iv, q6h, until reach safe serum MTX concentration, Dexamethasone 40mg/d ivdrip, d1-4.). For newly diagnosed stage I/II patients, both regimens were repeated every three weeks for a maximum four cycles as induction chemotherapy and followed by EIFRT at the dosage of 56Gy in 28 fractions over 4 weeks. Primary EIFRT was delivered using 6-MeV linear accelerator using 3-dimensional conformal treatment planning. For newly diagnosed stage III/IV or relapsed/refractory ENKTL, the regimens were repeated every three weeks for a maximum six cycles. Patients underwent autologous hematopoietic stem cell transplantation (ASCT) as consolidation if they achieved response (complete remission,CR or partial remission,PR). The primary endpoint was progression-free survival(PFS).
Results
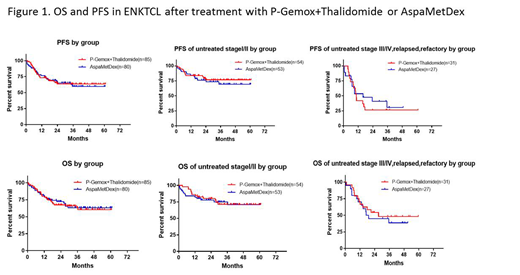
Between March 2014 and March 2018, 165 patients were randomly assigned. 85 patients to Group A, 80 patients to Group B. 156 patients were evaluable for response. Investigator-assessed overall response at the end of induction was 88.2% in the Group A and 75.0% in the Group B. Complete remission (CR) rate were 60.0% and 55.0%. Among 107 newly diagnosed stage I/II patients, 54 patients were assigned to Group A (52 assessed), and 53 to Group B (47 assessed). Overall response during induction in Group A and B was similar in both groups, were 64.8% and 64.2%. 58 newly diagnosed stage III/IV or relapsed refractory patients were enrolled. 31 patients were assigned to Group A (30 assessed), and 27 to Group B. The efficacy rate of Group A was higher than that of Group B. Overall response rate were 87.1% and 66.6%, respectively. At median follow-up of 24.6 (1.0-60.9)months, 3-year progression-free survival (PFS) and overall survival (OS) of whole cohort were 61.4% and 63.4%. PFS and OS rate of Group A were similar to Group B(Figure 1). Group B was better tolerated than Group A, with lower rates of agranulocytosis, thrombocytopenia and infections. While anemia,hyperbilirubinemia, edema, and increased BUN/Cr were more common in Group B. Three patients died of treatment related toxicity only in Group B . Two patients died of severe acute renal failure and sepsis at the first cycle, and one patient died of sepsis at the third cycle.
CONCLUSION:
Induction chemotherapy of both P-Gemox+Thalidomide and AspaMetDex regimen followed by EIFRT yielded promising efficacy for patients with stage I/II ENKTL. There is little difference therapeutic effect between the two regimens. For advanced or relapsed patients, both regimen showed unsatisfied survival outcome. Meanwhile, P-Gemox+ Thalidomide was less toxic with more convenient administration in outpatients clinics in comparison to AspaMetDex. ( ClinicalTrials.gov, NCT 2085655 ).
Li:Guangdong Province Hospital: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal