INTRODUCTION
The optimal frontline treatment for mantle cell lymphoma (MCL) is not clearly defined. Bendamustine + rituximab (BR) is commonly used as initial therapy. The role of maintenance rituximab (MR) after BR is not agreed upon due to limited data supporting this practice, whereas MR improves overall survival (OS) after autologous stem cell transplant (ASCT) and after R-CHOP for elderly patients who do not receive ASCT. Preliminary results from a subgroup analysis of the randomized phase 3 MAINTAIN study revealed neither a progression-free survival (PFS) nor OS benefit for MR as compared to observation for MCL pts (Rummel, ASCO 2016). In follicular lymphoma patients, however, there does appear to be a PFS benefit to rituximab maintenance following BR. Given these disparate results, we sought additional data to evaluate the role of rituximab maintenance following BR in MCL
METHODS
MCL pts treated at 12 U.S. medical centers with frontline BR who achieved a complete response (CR) or partial response (PR) and who did not receive consolidative ASCT from 2011 - 2017 were included. Use of MR was based on individual physician/patient preferences. Baseline pt characteristics were compared using chi-squared test, Fisher's exact tests, or ANOVA. Descriptive statistics, comparisons, and OS using the Kaplan-Meier method were stratified by response status as determined by the treating site (complete response (CR) only, partial response (PR) only, and CR/PR).
RESULTS
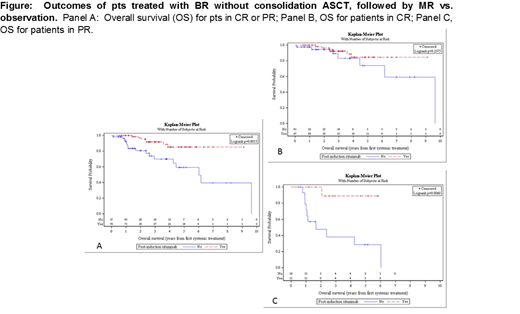
Among 135 pts responding to frontline BR who did not complete subsequent ASCT, 80% achieved complete remission (CR) and 20% had a partial remission (PR). Median age was 70 (range 45 - 93) years and 66% were male. Baseline MIPI score was low (13%), intermediate (38%), or high (49%) among patients with available data (n = 92) and did not differ between treatment cohorts. Among responding patients, 78 (58%) received MR and 57 (40%) were observed. With a median follow up of 3.1 years, median OS was not reached for pts responding to BR (with CR or PR) who received MR vs. 6 years for those who received no maintenance (Figure Panel A, P = 0.0013). Use of MR vs. observation was associated with a significant improvement in OS for pts in PR at the end of induction therapy (Figure Panel B, median not reached vs. 1.7 years, P = 0.006), but there was no statistically significant OS difference for pts in CR (Figure Panel C, median not reached vs. 9.6 years, P = 0.2575). In multivariable analysis, MIPI score <6.2 was associated with improved OS [Hazard Ratio (HR) 0.35 (95% confidence interval (CI) 0.12 - 1.02], P = 0.055) as was the use of MR [HR 0.28 (95% CI 0.10-0.79), P = 0.016].
CONCLUSIONS
Whereas the MAINTAIN trial showed no PFS advantage to MR after BR for MCL pts, in this multi-center outcomes analysis across multiple US centers, the use of MR was associated with an improvement in OS in pts receiving frontline BR without consolidative ASCT. The survival benefit was only observed for pts in PR after induction therapy. Because of the potential for selection bias in the application of MR, further validation cohorts and prospective study are needed to clarify if there is benefit to maintenance therapy after BR-treated patients not undergoing ASCT as well as the potential for differential benefit of maintenance based on remission status
Hill:Takeda: Research Funding; Amgen: Research Funding; Celegene: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; TG therapeutics: Research Funding; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Consultancy, Research Funding. Martin:Janssen: Consultancy; I-MAB: Consultancy; Celgene: Consultancy; Sandoz: Consultancy; Karyopharm: Consultancy; Teneobio: Consultancy. Calzada:Seattle Genetics: Research Funding. Kolla:Amgen: Equity Ownership. Bachanova:Gamida Cell: Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Kite: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; GT Biopharma: Research Funding; Novartis: Research Funding. Gerson:Abbvie: Consultancy; Seattle Genetics: Consultancy; Pharmacyclics: Consultancy. Barta:Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Celgene: Research Funding; Merck: Research Funding; Mundipharma: Honoraria; Seattle Genetics: Honoraria, Research Funding; Celgene: Research Funding; Mundipharma: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy, Research Funding. Danilov:Curis: Consultancy; Bristol-Meyers Squibb: Research Funding; TG Therapeutics: Consultancy; Abbvie: Consultancy; Genentech: Consultancy, Research Funding; Bayer Oncology: Consultancy, Research Funding; Takeda Oncology: Research Funding; MEI: Research Funding; Pharmacyclics: Consultancy; Aptose Biosciences: Research Funding; Verastem Oncology: Consultancy, Other: Travel Reimbursement , Research Funding; Seattle Genetics: Consultancy; AstraZeneca: Consultancy, Research Funding; Gilead Sciences: Consultancy, Research Funding; Celgene: Consultancy; Janssen: Consultancy. Grover:Seattle Genetics: Consultancy. Karmali:Takeda, BMS: Other: Research Funding to Institution; Astrazeneca: Speakers Bureau; Gilead/Kite; Juno/Celgene: Consultancy, Speakers Bureau. Ghosh:Celgene: Consultancy, Research Funding, Speakers Bureau; T G Therapeutics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Speakers Bureau; Spectrum: Consultancy, Speakers Bureau; Astra Zeneca: Speakers Bureau; Forty Seven Inc: Research Funding; Genentech: Research Funding; Gilead/Kite: Consultancy, Speakers Bureau; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Park:G1 Therapeutics: Consultancy; Rafael Pharma: Membership on an entity's Board of Directors or advisory committees; Teva: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Gilead: Speakers Bureau; Seattle Genetics: Research Funding, Speakers Bureau. Epperla:Pharmacyclics: Honoraria; Verastem Oncology: Speakers Bureau. Hamadani:Otsuka: Research Funding; Merck: Research Funding; Celgene: Consultancy; ADC Therapeutics: Consultancy, Research Funding; Janssen: Consultancy; Sanofi Genzyme: Research Funding, Speakers Bureau; Pharmacyclics: Consultancy; Medimmune: Consultancy, Research Funding; Takeda: Research Funding. Kahl:Seattle Genetics: Consultancy; BeiGene: Consultancy; TG Therapeutics: Consultancy; ADC Therapeutics: Consultancy, Research Funding. Flowers:Optimum Rx: Consultancy; Acerta: Research Funding; Gilead: Consultancy, Research Funding; Genentech, Inc./F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; National Cancer Institute: Research Funding; TG Therapeutics: Research Funding; AbbVie: Consultancy, Research Funding; Bayer: Consultancy; Eastern Cooperative Oncology Group: Research Funding; Millenium/Takeda: Research Funding; V Foundation: Research Funding; Karyopharm: Consultancy; BeiGene: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Burroughs Wellcome Fund: Research Funding; AstraZeneca: Consultancy; Pharmacyclics/Janssen: Consultancy, Research Funding; Spectrum: Consultancy; Denovo Biopharma: Consultancy. Cohen:Janssen Pharmaceuticals: Consultancy; Seattle Genetics, Inc.: Consultancy, Research Funding; Bristol-Meyers Squibb Company: Research Funding; Takeda Pharmaceuticals North America, Inc.: Research Funding; Gilead/Kite: Consultancy; LAM Therapeutics: Research Funding; UNUM: Research Funding; Hutchison: Research Funding; Astra Zeneca: Research Funding; Lymphoma Research Foundation: Research Funding; ASH: Research Funding; Genentech, Inc.: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal