Physiologically T-regulatory cells (Treg) suppress immune responses against self-antigens preventing autoimmunity. However, in cancer they are believed to suppress anti-tumor immune response, and the presence of Treg in the tumor microenvironment has been associated with adverse outcome in most cancers. Treg are integral part of tumor microenvironment in lymphoma, and can use different mechanisms to inhibit both adaptive and immune response (including CD4, CD8, DC, macrophages, B-cells and NK cells) modulating therefore the interaction between lymphoma and the host microenvironment. Increased numbers of Treg have been associated with adverse clinical outcome in follicular lymphoma (FL) but a more favorable outcome in classical Hodgkin lymphoma (CHL). In this study we examined Treg phenotype in detail using multiparameter flow cytometry in lymph node specimens with normal histology (NLN) and involved by FL and CHL. We report that both CD4 positive T-cells and Treg subset show distinct phenotypes in different disease entities suggesting different biological functions.
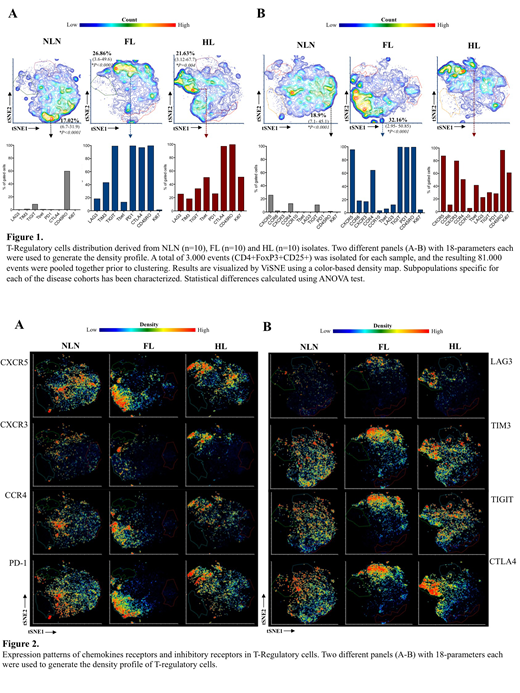
Single cell suspensions were prepared from NLN (n=10) and lymph node biopsies involved by FL (n=10) and CHL (n=10), and high dimensional multiparameter flow cytometric immunophenotyping was performed using antibodies against immune checkpoints (TIGIT, TIM3, PD-1, CD96, LAG3, CTLA4, CD73). To characterize the CD4 T-cell compartments we used a dimensionality reduction algorithm for non-linear data representations (tSNE). We studied the CD4+FoxP3-CD25- and Treg characterized by CD4+FoxP3+CD25+ phenotype separately and in both the compartments we identified and characterized subpopulations specific for each of the disease cohorts.
tSNE representations CD4 positive T-cells and Treg showed different distributions in NLN, FL and HL. In NLN, CD4 cells broader heterogeneity without distinct clusters whereas in FL and CHL CD4 positive T-cells and Treg (Figure 2 A-B) showed highly polarized phenotypes which were distinct from each other and nearly absent within normal Treg compartment.
We observed a strong expression of the immune checkpoint regulators TIM3, LAG3, CTL4, Tbet and PD1 in CD4 T-cells derived from HL tissues. In contrast FL CD4 T-cells were mainly characterized by an up regulation of PD1, CTLA4 and TIGIT. The TIGIT+ cells in FL samples are mainly CXCR5+PD1bright and they up regulate CTLA-4 in the FoxP3+ compartment. In all the neoplastic tissues the FoxP3+CD25+ T-Reg express mainly an activated/memory phenotype (CD45RO+CTLA4+), but while in HL microenvironment they show a TH1 phenotype (CXCR3+Tbet+), in FL they mainly express PD1+CXCR5+ (Figure 1B). Additionally, the dominant population of regulatory T-cells derived from HL samples down modulate PD1 expression, show high level of expression of TH1-associated transcription factor Tbet and have high proliferation index, while in FL, PD1 is brightly expressed, Tbet is not expressed and proliferation index is low in the dominant population (Figure 1A).
The distinct phenotypic differences of Treg in FL lymphoma and CHL may account for the better prognosis seen in CHL with increased Treg which has TH1 like phenotype, therefore predicted to have anti-tumor activity. The differences seen in the expression immune checkpoint regulators both on CD4 positive T-cells and Treg subset may explain different rate responses seen in FL compared to CHL with checkpoint therapy.
Roshal:Physicians' Education Resource: Other: Provision of services; Celgene: Other: Provision of Services; Auron Therapeutics: Equity Ownership, Other: Provision of services. Dogan:Corvus Pharmaceuticals: Consultancy; Novartis: Consultancy; Takeda: Consultancy; Roche: Consultancy, Research Funding; Celgene: Consultancy; Seattle Genetics: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal