Introduction: Anti-CD20 antibody drugs such as rituximab (RTX) and obinutuzumab (OBZ) are used ubiquitously in the treatment of B cell NHL. However, therapeutic resistance to anti-CD20 antibodies occurs quite commonly, especially in indolent NHL. Known mechanisms of resistance include loss of CD20 expression, poor immunoreactivity and dysfunctional apoptotic responses. However, few therapeutic strategies have been established to overcome resistance. Identification of new, targetable anti-CD20 resistance mechanisms are needed.
Methods: Anti-CD20 RTX resistant (RR) and OBZ resistant (OR) cells were developed using SUDHL4 and SUDHL10 cultured with chronic low antibody (Ab) drug concentrations. Development of anti-CD20 resistance were characterized by CD20 immunophenotyping, gene expression profiling and systems biology analysis. Natural killer (NK)-mediated ADCC activity (AcellaTox-Glo) with real-time interaction kinetics by microfluidics was utilized for immunological characterization of anti-CD20 resistance. Calcium agonists (ionomycin, thapsigargin & PMA) inducible calcium release was evaluated using Fluo-4 cell based assay. Western blot analysis was used to investigate BCR, autophagy and proteosomal signaling pathways. Metabolomic profiling was performed using Mass Spectrometry.
Results: Microfluidic analyses of co-encapsulated single NK and SUDHL10 cell 1:1 effector-target (E:T) ratio revealed that NK cells engaged briskly with target cells within <50 minutes and resulted in >75% cell death within 120 minutes in the presence of anti-CD20 Ab drugs in RTX & OBZ-sensitive cells (P<0.001). These interaction kinetics indicated that CD20 Ab drugs facilitated rapid engagement & sustained tumor-NK interactions to promote cell death. However, ADCC assays using anti-CD20 Abs and primary NK cells revealed a marked decrease in NK mediated ADCC activity in RR (11%, P=0.0002) and OR (17%, P=0.001) cells compared with RTX- or OBZ-sensitive NHL B cells. Transcriptomic and secretomic analyses revealed downregulation of calcium dependent inflammatory responses (i.e., IFN, NFAT, IL6, IL22 & complementary pathways) with concurrent upregulation of nucleotide metabolism in both RR and OR cells.
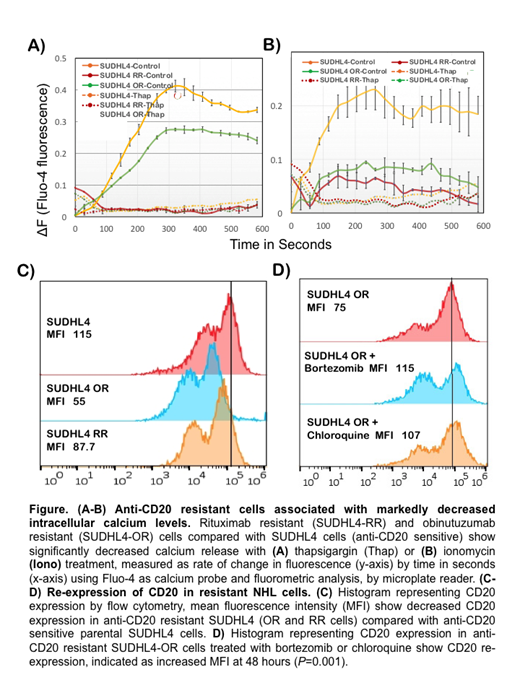
Subsequently, analysis for 'store operated' calcium demonstrated lack of thapsigargin-releasable calcium in anti-CD20 resistant cells (Fig A-B) resulting in significant depolarization of NHL cells. While depolarization is relevant to BTK activation and mitogenic responses, we also observed activation of the pro-survival pathway, JNK (by phosphorylation), corresponding to lower calcium levels in anti-CD20 resistant NHL cells. Lower calcium levels also modulated proteasomal function and promoted glucose-dependent metabolic activity. Additionally, metabolic profiling identified increased glucose uptake and significant increases in the pool sizes of AMP, GMP, CMP and UMP in both RR and OR cells compared with parental SUDHL-4 cells, which validated increases in nucleotide metabolism as predicted by transcriptomic studies.
Thus, we hypothesized that calcium-dependent mechanisms may be exploited to induce cell death in anti-CD20 resistant cells. We delineated the impact of reversing depolarization with ivermectin, inhibition of BTK with acalibrunitib, and blocking autophagy and proteasomal function using chloroquine and bortezomib, respectively, in RR & OR cells. Findings indicated that co-targeting with acalibrunitib & ivermectin resulted in significantly decreased cell viability in SUDHL-4 RR cells compared with parental SUDHL4 cells (P<0.0001). Furthermore, chloroquine or bortezomib treatment resulted in increased CD20 expression both in parental and anti-CD20 resistant cells (Fig C-D). In vivo studies of these therapeutics alone & combined are under evaluation in CD20 sensitive and resistant human xenograft NHL models and will be reported at the meeting.
Conclusions: We identified calcium as a critical modulator of anti-CD20 resistance in B cell NHL. Furthermore, rational multi-targeting of cellular voltage potential with BTK signaling, autophagy, and proteasomal activity resulted in CD20 re-expression and prominent enhancement of cell death in resistant NHL cells. Collectively, these data suggest that small molecule compounds targeting ionic signaling, many of which are already approved for human use, may be harnessed as novel cancer therapies.
Evens:Pharmacyclics: Consultancy, Honoraria; Verastem: Consultancy, Honoraria; Tesaro: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding; Epizyme: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal