Introduction
Cutaneous T-cell lymphomas (CTCLs) are characterized by dermal and epidermal infiltration of skin homing clonal CD4+ memory T-cells. Little is known about the oncogenic events driving either the progression of skin-limited disease such as Mycosis Fungoides (MF) to a leukemic form or Sézary Syndrome (SS), and there are no histologic means to predict evolution. Genetic instability is a hallmark of malignancy progression and telomere remodeling has been shown to play a role in the progression of hematological malignancies. Thus, the aim of this study is to characterize the three-dimensional (3D) telomeric organization in early and advanced CTCLs.
Methods
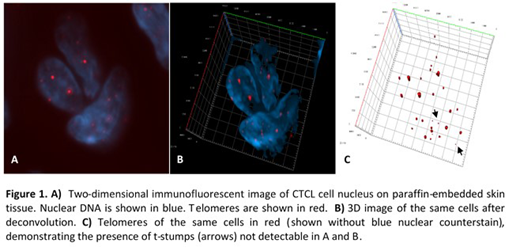
We performed 3D telomeric quantitative fluorescent in situ hybridization (3D Telo-Q-FISH) of 5mm skin tissue slides of 10 patients with MF and SS and of CD4+ lymphocytes of 3 healthy controls (Figure 1). Using the program TeloView (Vermolen et al., 2005), the proportion of telomeres of low intensity (TLI) (<5000 u), the nuclear volume and the total number of telomeric signals per cell were calculated. Patients were stratified based on CD30 expression (CD30 high, n=3 versus CD30 low, n=7) and clinical stage (early stages I-IIA, n=6 versus advanced stages IIB-IV, n=4).
Results
TLI represented 27% of telomeres in CTCL cells compared to 16% in control lymphocytes (p<0.0001). The highest proportion of TLIs was found in tumors CD30 high (34%), compared to 22% in tumors CD30 low (p<0.0001). Similar findings were observed when stratifying for advanced and early clinical stages (30% vs 24%, p<0.0001). Nuclear volume and total number of telomeric signals increased in CTCL cells expressing CD30 (both p<0.0001), and in CTCL cells associated with advanced clinical stages (p=0.0021 and p=0.001, respectively). However, as expected, nuclear volume and total number of telomeric signals were higher in control cells compared to CTCL cells (both p<0.0001).
Conclusion
We report clear evidence that CTCLs with either CD30 expression or advanced clinical stage are associated with loss of telomeres and telomeric signal intensity. These very small telomeres, termed 't-stumps', are a hallmark feature in many tumor cells. Analysis of the nuclear volume and total number of telomeric signals suggest that CTCL cells undergo in a first step telomere shortening and loss compared to healthy controls. In a second step further telomeric shortening associated with chromosomal rearrangements and bridge-breakage-fusion cycles may be involved in the progression of CTCL.
Pehr:Merck: Consultancy, Other: Clinical research on Alzheimers Drug; Eli Lilly: Consultancy, Other: Clinical research on Alzheimers Drug; galderma: Consultancy, Other: tactupump Forte; Actelion: Consultancy; valeant: Consultancy; CeraVe: Consultancy; Janssen-Cilag: Other: Train the trainer.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal