Background: CLR 131 is a novel targeted radiotherapeutic that exploits the selective uptake and retention of phospholipid ethers (PLEs) by malignant cells. Based on initial preclinical and clinical experience and the radiosensitivity of MM, fractionated dosing of CLR 131 is being examined in RRMM in a Phase 1 trial (NCT02278315) and a Phase 2 trial, CLOVER-1 (NCT02952508).
Methods: Both the Phase 1 and Phase 2 trials of CLR 131 aim to determine efficacy and safety in RRMM. Eligibility criteria include progressive or relapsed MM that is refractory to at least 1 proteasome inhibitor (PI) and 1 immunomodulatory (IMiD) drug with no upper limit to the number of prior lines of therapy. Prior autologous stem cell transplant (ASCT) and external beam radiation therapy are allowed (< 20% of total marrow irradiated). The Phase 1 trial was a single and fractionated ascending dose escalation safety study and the Phase 2 trial is evaluating 3 doses: a bolus dose and 2 fractionated doses. The fractionated doses of CLR 131 included infusion of either 31.25 mCi/m2 CLR 131 or 37.5 mCi/m2 CLR 131 (administered as 15.625 mCi/m2 or 18.75 mCi/m2, respectively, on day 1 and day 7 (± 1 day)) administered as a 30-minute intravenous infusion is reported here. Adverse events (AEs) are graded by NCI-CTCAE v4.03. Responses were determined using IMWG criteria as assessed by the investigator.
Results: As of 30Jul2019, 10 subjects have received fractionated 31.25 mCi/m2 and 6 subjects fractionated 37.5 mCi/m2 CLR 131. In addition, 1 subject was scheduled to receive fractionated 37.5 mCi/m2 CLR 131 but died due to progressive disease prior to administration of the second dose; this subject is not included in the analyses below as they did not receive both fractionated doses. There is no upper age limit for enrollment and the median age for the 16 RRMM patients was 71 (range 51-83), including 6 females and 10 males with a median of 4 prior therapies (range 2 to 13). Seven patients had prior ASCT.
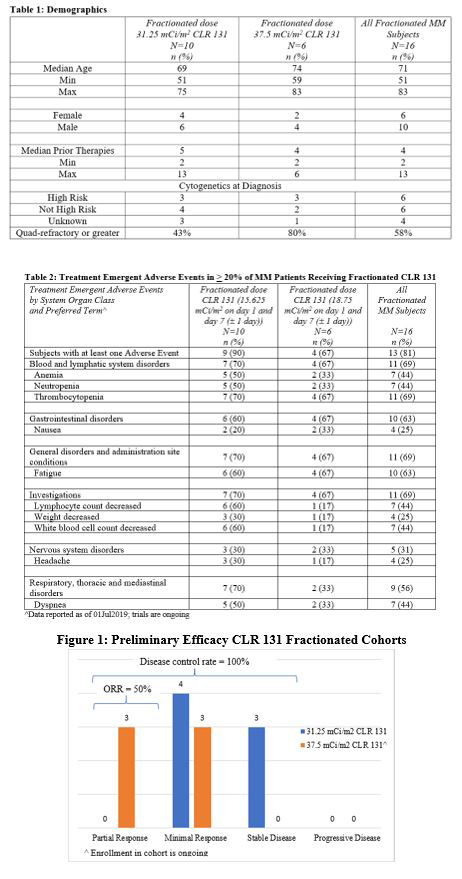
CLR 131 demonstrated 100% disease control rate in subjects receiving either fractionated dose of CLR 131. The overall response rate (ORR) in the fractionated 37.5 mCi/m2 cohort is 50%. Three subjects in the cohort experienced a partial responses (PR), median time to response 43 days, and the other three had minimal responses (MR) with an average 39% reduction in m-protein. In this cohort, 80% of the subjects were either quad- or penta-refractory; all 80% were refractory to daratumumab. There were two subjects in the 31.25 mCi/m2 cohort with non-secretory disease and their status was followed by FDG-PET imaging. Both have been excluded from the evaluation of efficacy as their disease does not meet with IMWG criteria for response. No patients in this cohort achieved a PR or better however a majority of the subjects experienced a minimal response. The primary AEs include thrombocytopenia, anemia, neutropenia, and fatigue (Table 2). The hematologic AEs were expected, manageable and followed a predictable timeline to nadir (average. 40 days) and subsequent recovery (average 17 days post nadir).
Conclusions: CLR 131 is a unique, first in class targeted radiotherapeutic for RRMM. Preliminary data for CLR 131 administered as a fractionated dose shows an acceptable and expected safety profile in this patient population. Fractionated dosing at 37.5 mCi/m2 has shown an efficacy signal and has been adopted as the standard for CLR 131 dosing in ongoing and future trials. Dose escalation to determine the highest tolerated dose is ongoing in the Phase 1 study and is currently examining a fractionated infusion of 40 mCi/m2 administered as 20 mCi/m2 CLR 131 on day 1 and day 7 (± 1 day). Based upon these data enrollment to the fractionated 37.5 mCi/m2 cohort of the Phase 2 trial continues.
Ailawadhi:Celgene: Consultancy; Amgen: Consultancy, Research Funding; Pharmacyclics: Research Funding; Cellectar: Research Funding; Janssen: Consultancy, Research Funding; Takeda: Consultancy. Stiff:Gilead/Kite Pharma: Consultancy, Honoraria, Research Funding; Amgen: Research Funding; Gamida-Cell: Research Funding; Incyte: Research Funding; Cellectar: Research Funding; Unum: Research Funding. Ibrahim:Cellectar: Honoraria, Research Funding; Incyte: Research Funding; Pfeizer: Research Funding; Puma: Research Funding; Eli Lilly: Research Funding; Hoffman-LaRoche: Research Funding; Spectrum: Research Funding; Takeda: Research Funding. Cull:Celgene: Speakers Bureau; ADC Therapeutics: Research Funding. Green:GSK: Consultancy; Seattle Genetics: Research Funding; Juno Therapeutics: Consultancy, Patents & Royalties, Research Funding; Celgene: Consultancy; Cellectar: Research Funding. Oliver:Cellectar Biosciences: Employment. Longcor:Cellectar Biosciences: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal