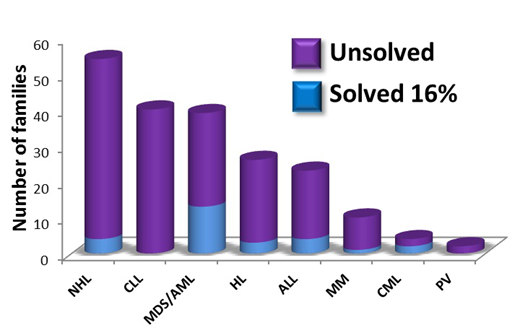
The Australian Familial Haematological Cancer Study (AFHCS) was initiated in 2004 with the aim to define genes predisposing to hematological malignancy (HM) to offer better options for clinical decision making and genetic counselling, and to identify therapeutic targets. The study is a referral centre for Australia and New Zealand, and currently has 230 families with multiple cases of myeloid and/or lymphoid malignancies or early onset cases (Figure 1), and is growing as clinical awareness of a germline genetic basis for blood cancers increases.
To date, we have identified families with causal germline variants in several predisposition genes (five GATA2, ten RUNX1, one CEBPA, ten DDX41, one SAMD9L) including novel single nucleotide variants, deletions and insertions in coding and intronic sequences using traditional Sanger sequencing and now genomic and transcriptomic technologies. Of these, one GATA2 and four DDX41 germline mutations were identified during the screening of "sporadic" MDS samples. All four DDX41 mutant samples also harbored a somatic DDX41 (R525H) variant on the other allele at a low variant allele frequency. A comprehensive clinical analysis of the RUNX1 families has uncovered segregating phenotypes, in addition to thrombocytopenia and myeloid and lymphoid malignancies, including skin disorders such as psoriasis. In an increasing number of individuals in these families, important clinical decisions have been made dependent on mutation carrier status.
Recently, we have identified and characterized a unique myeloproliferative neoplasm (MPN)/acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS) family with a germline Chr14q duplication that overlaps with duplications in two other reported MPN/AML families. This appears to be a unique genotypic/phenotypic entity when compared to other myeloid predisposition genes and their associated phenotypes.
Interestingly, we have identified several families carrying heterozygous pathogenic/likely pathogenic variants in genes representing autosomal recessive genomic instability syndromes segregating with HM. Here mutations in the genes NBN, RECQL4, DDX11 and RAD21 appear to act in an autosomal dominant manner. Further, we have found DNA damage repair gene predicted pathogenic variants in PALB2 and BARD1 in families with both solid cancers and HM, predominantly lymphomas, implicating an expansion of the major predisposition phenotype of these gene perturbations.
Familial cases of chronic lymphocytic leukemia (CLL) have been well recognized, but it has been particularly difficult to identify predisposing variants. We have identified a number of strong candidate genes/variants in CLL families including PRPF8 (Y208C and N400S) and SAMHD1 (R371H) although more families are required to confirm these.
An integral part of the AFHCS is the continued generation of cell and animal models to help define mechanisms of action of predicted or known pathogenic variants, and functional model systems for testing of variants of unknown significance. To facilitate the collection of patient samples, we have adopted the use of hair bulbs as the main germline sample as they are easy to collect, can be easily sent long distance by mail at room temperature, require no culture, are quickly and cheaply processed and provide good quality DNA using automated procedures.
Overall, collaborative efforts within Australia and New Zealand and internationally have been highly fruitful in solving familial cases of hematopoietic malignancies over the last 15 years, and even more concerted international efforts will be required in the future to uncover the familial basis of unsolved cases, particularly in the lymphoid lineage, and to clarify best approaches for clinical decision making and treatment options.
Figure 1. Summary of AFHCS families with associated hematological malignancies.
Scott:Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal