There is growing appreciation that immune system plays an important role in anti-leukemia effect in patients with acute myeloid leukemia (AML). However, comprehensive illustration of phenotypic and functional immune signatures of AML at diagnosis is still lacking. Here, we conducted a prospective study to perform a comprehensive peripheral blood immune profiling through multiparameter flow cytometry and investigated prognostic immune-related risk factors in AML patients.
In cohorts of 50 newly-diagnosed patients and 24 healthy controls, we performed phenotypic and functional analysis of circulating lymphocytes including CD4+ T, CD8+ T, natural killer (NK), natural killer-liked T (NKT), gamma delta T (γδ T) and B cells to elucidate the nature of immune defects in AML development. Our findings suggest that immune defects in AML patients are operative in T and NK cells that are important component of antitumor immunity while B cell function remains unaffected. Both CD8+ T and CD4+ T cells from AML patients exhibit features of senescence and exhaustion at diagnosis. These terminally senescent and exhausted T cells are phenotypically characterized by increased expression of CD57 and PD-1 and decreased expression of CD28. In addition, lessened frequency of NK, NKT and γδ T cells indicate slack innate immunity in AML. One important parameter involved in NK cell function is the maturation stage, and we found similarly increased NK cell maturation in AML. NK cell dysfunction was further supported by downregulation of activating receptor NKG2D and NKP30, which may weaken the recognition and interaction between NK cells and AML blasts. Furthermore, diseased γδ T cells demonstrate a highly-activated or even exhausted state through a profound PD-1 upregulation and NKG2D downregulation.
We next performed immunologic studies on paired pre- and post-chemotherapy samples to analyze whether immune dysfunction was recovered during the course of induction chemotherapy. Immune reconstitution occurs only in patients with therapeutic response. T and NK cell function restoration is principally reflected in recovered proportions, decreased extent of terminal differentiation and partly improved T cell senescence and exhaustion. Moreover, we extended studies to analyze the difference in immune signatures between CR group (achieved stable complete remission after chemotherapy, n = 24) and RR group (not achieved complete remission after chemotherapy or suffered a relapse, n = 20). Patients in RR group exhibit selectively more senescent and exhausted CD8+ T (CD28lo/CD57hi/PD-1hi) and CD4+ T (PD-1hi) cells, lower percentages of γδ T cells and excessive NK maturation state.
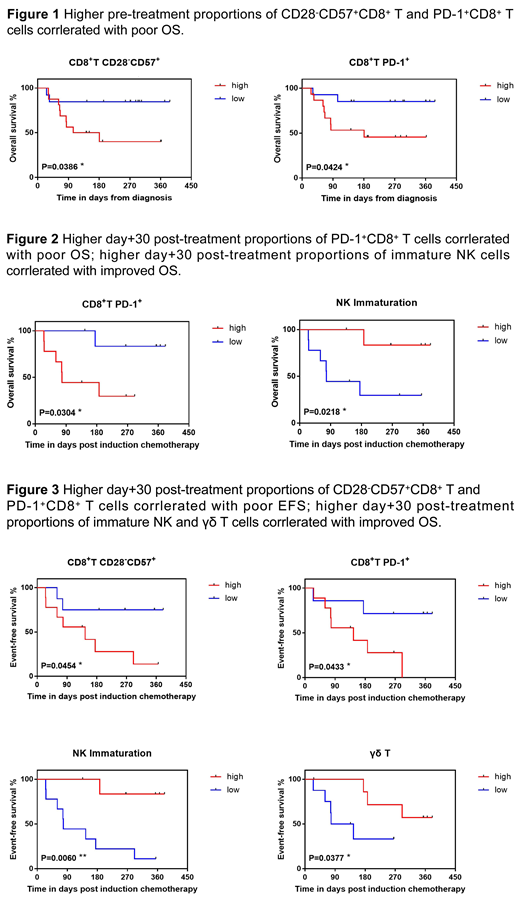
More importantly, we demonstrated that specific immune phenotypes apparently correlate with overall survival (OS) and event-free survival (EFS, the "event" refers to progression, relapse and death) in AML patients. Median values of the complete cohort were used for grouping the patients. Poor OS correlated with select pre-chemotherapy circulating PD-1+CD8+ and CD28-CD57+CD8+ T subsets (Figure 1). Elevated day +30 post induction chemotherapy PD-1+CD8+ T frequency and increased NK cell maturation correlated with unfavorable OS and EFS after induction chemotherapy. Moreover, decreased day +30 post induction chemotherapy CD28-CD57+CD8+ T and increased γδ T cell proportion were associated with improved EFS, but not with OS (Figure 2 and Figure 3).
This is the first study to provide insights into longitudinal immunological landscape at diagnosis and after chemotherapy in patients with AML. Select immune phenotypes correlate with EFS and OS, implying that non-invasive immune testing of blood samples can be applied to identify high risk for relapse and unfavorable prognosis. Personized immunotherapies, aimed at reactivating senescent T cells, reverting exhausted T cells or improving innate NK and γδ T immunity, may contribute to better therapeutic effects and reduced early relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal