Introduction: IMGN632, a novel CD123-targeting ADC, demonstrates a favorable safety profile and complete remissions as a monotherapy in patients with relapsed/refractory AML and BPDCN (NCT03386513). We have previously reported the synergy of combining IMGN632 with venetoclax (BCL-2 inhibitor) (EHA, 2019, abstract #PF201). Given the recent approval of azacitidine (AZA, a hypomethylating agent) and venetoclax (VEN) in AML patients unfit for intensive chemotherapy, we investigated the combination of IMGN632 with AZA and the triple combination of IMGN632, AZA and VEN.
Methods: The IMGN632 and AZA +/- VEN combinations were evaluated in the following AML models: EOL-1, subcutaneous (SC); Molm-13, disseminated; MV4-11, disseminated; and one patient derived AML xenograft (PDX) model. In SC models, tumor volume was measured serially, complete regressions (CRs) were noted, and endpoint was determined by either clinical observations or when tumor volume reached 1000 mm3. Tumor growth inhibition (T/C) was calculated as (median tumor volume of the treated / median tumor volume of the control) x 100%. In disseminated models, the percent Increased Life Span (%ILS) was calculated as [(T-C, tumor growth delay) / C] x 100%, where T is the median survival time (days) of a treated group and C is the median survival time (days) of the vehicle control group. In AML PDX models, tumor burden was monitored by hCD45/hCD123 flow cytometry of serial peripheral blood (PB) samples.
Results: In EOL-1, treatment with AZA (3 mg/kg, daily x5) was inactive, producing only a 4% reduction in T/C and 0/6 CRs treatment. IMGN632, at a sub-optimal single dose of 0.08 mg/kg resulted in a 36% reduction in T/C and 2/6 long-term CRs at study end. The combination of IMGN632 and AZA generated a robust 100% reduction in T/C and 4/6 CRs, while addition of VEN (100 mg/kg, daily x28) to AZA was not as active with a 54% reduction in T/C and 1/6 CRs. The triple combination with the addition of IMGN632 to the VEN + AZA doublet was the most active, with a 100% reduction in T/C and 5/6 CRs.
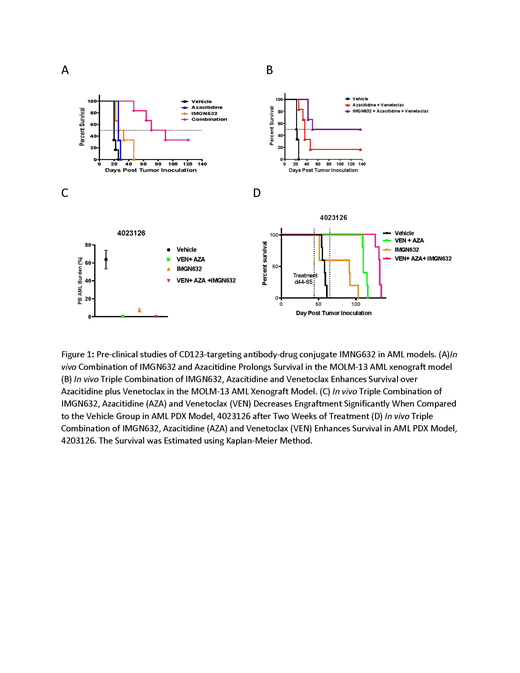
Similarly, in Molm-13, AZA (3.5 mg/kg, daily x5) was minimally active, with in a 6-day T-C and 30% ILS. IMGN632 (0.024 mg/kg, weekly x3) was active and generated a 9.5-day T-C and a 47.5% ILS. Strikingly, the combination of IMGN632+AZA was highly active, resulting in a 61-day T-C and a 303% ILS (Figure 1A), demonstrating a clear survival benefit over single agent treatments. In a separate Molm-13 study, the combination of AZA+ VEN produced a 14.5-day T-C and a 67.4% ILS (highly active), while the triple combination including IMGN632 was clearly the most active combination, with a >116-day T-C and a >537% ILS (highly active) 50% of mice surviving beyond 4.5 months (Figure 1B).
Similarly, in the MV4-11 model, the two AZA (either 4.5 mg/kg or 3 mg/kg, daily x5) + VEN (100 mg/kg, daily x28) combinations resulted in a 28-day T-C (62.2% ILS) and a 20-day T-C (44.4% ILS), respectively. The addition of IMGN632 (0.08 mg/kg) to these resulted in a superior regimen with a 64-day T-C (142.2% ILS) and a 76-day T-C (168.9% ILS), respectively.
The PDX AML model, 4023126, responded very well to treatment with the triple combination (AZA (2.5mg/kg, daily x 5), VEN (100 mg/kg, 5 days/week x3 weeks) and sub-optimal IMGN632 (0.12 mg/kg once a week x3 weeks), with circulating leukemia burden lower than 1% after two weeks of treatment, compared to greater than 50% in the vehicle-treated group (Figure 1C). In model 4023126, IMGN632 was active and resulted in a 33-day T-C and 57% ILS, and AZA+VEN resulted in a 52-day T-C and 90% ILS. The triple combination group survived the longest and was highly active resulting in a 74-day T-C and 128% ILS demonstrating highest survival benefit (Figure 1D).
Conclusions: The addition of IMGN632 to AZA alone and to AZA+VEN in multiple AML xenograft and PDX models leads to improved survival. These data support the addition of a CD123-targeted ADC with a novel DNA damaging payload to standard of care AZA and AZA+VEN in AML patients
Kuruvilla:The University of Texas M.D.Anderson Cancer Center: Employment. McCarthy:ImmunoGen: Employment. Zhang:The University of Texas M.D.Anderson Cancer Center: Employment. Sloss:ImmunoGen: Employment. Zweidler-McKay:ImmunoGen: Employment. Romanelli:ImmunoGen: Employment. Konopleva:Calithera: Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Astra Zeneca: Research Funding; Agios: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal