Background: Acute myeloid leukemia patients with FLT3-ITD mutations have a high risk of relapse and death. FLT3 tyrosine kinase inhibitors such as quizartinib and gilteritinib improve overall survival in relapsed patients, but their efficacy is limited and most such patients die of the disease. This is because even with potent FLT3 inhibition, the disease persists within the bone marrow microenvironment, mainly due to bone marrow stroma activating parallel signaling pathways that maintain pro-survival factors. BET inhibitors suppress pro-survival factors such as c-Myc and Bcl2, but these drugs thus far have shown only limited single-agent clinical potential. PLX51107 is a novel BET inhibitor designed to inhibit BET activity in intermittent daily fashion to allow for greater tolerability. We investigated whether the addition of PLX51107 to potent FLT3 inhibition with quizartinib could overcome the protective effect of the bone marrow stroma through inhibition of c-Myc expression.
Methods: We developed a plasma inhibitory activity assay for c-Myc (c-Myc PIA) to assess in vivo efficacy of the PLX51107 in patients and to identify c-Myc-inhibitory doses of the drug. We tested PLX51107 alone and in combination with quizartinib in murine models of AML and against primary FLT3-ITD AML cells co-cultured with human bone marrow stroma. In addition, we analyzed gene expression patterns in the treatment models to explore the basis of any observed synergistic cytotoxic effect.
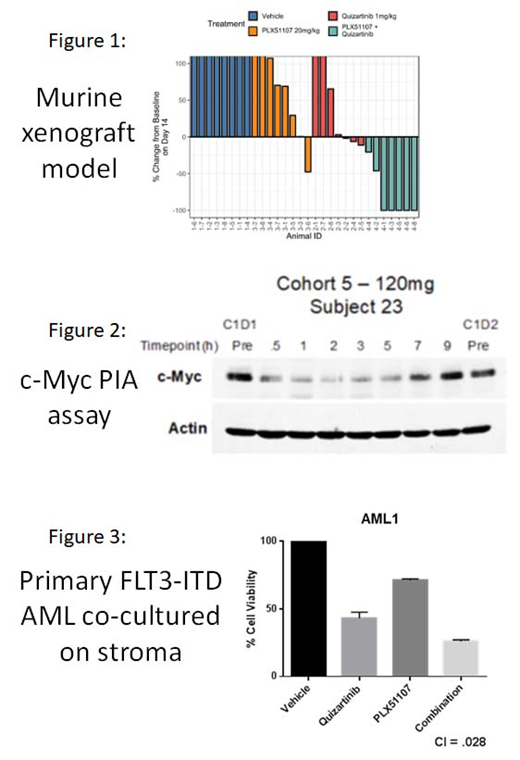
Results: In a murine xenograft model of AML using MV4-11 cells, PLX51107 alone induced suppression of tumor growth in association with a 90% decrease in c-Myc gene expression. The combination of PLX51107 and quizartinib induced complete tumor regression in 5 out of 7 animals after 14 days of treatment (Figure 1). Animals treated with a 5-day course of quizartinib alone displayed tumor regression persisting until day 26 of treatment, while the addition of PLX51107 resulted in tumor regression until day 39. In patients treated with a dose of 120 mg/day of PLX51107, the c-Myc PIA demonstrated a robust suppression of c-Myc expression for roughly 6 hours, returning to baseline between 7 and 9 hours post-treatment (Figure 2). The mean plasma concentration to achieve this inhibition was 3.3 uM, which, accounting for the difference in protein drug binding between plasma and culture medium with 10% FBS, corresponded to a concentration of 250 nM PLX51107 in culture medium. With this same concentration and schedule (i.e., at concentrations and exposure times equivalent to what human patients would experience taking 120 mg daily of PLX51107 and 60 mg daily of quizartinib), the combination induced synergistic cytotoxicity in a series of 10 different FLT3-ITD AML patient blast samples co-cultured with bone marrow stroma (Figure 3). C-Myc RNA and protein were directly suppressed in these primary samples, and ingenuity pathway analysis of RNA expression confirmed that c-Myc associated genes displayed the highest level of down-regulation.
Conclusions: These studies suggest that combination therapy with approximately 120 mg/day PLX51107 and 60 mg/day quizartinib will be a more effective therapy for relapsed FLT3-ITD AML than 60 mg/day of quizartinib alone. The combination of FLT3 inhibition and BET inhibition may represent an attractive therapeutic option for FLT3-ITD AML.
Hizukuri:Daiichi Sankyo Co, Ltd: Employment. Severson:Plexxikon Inc.: Employment. Powell:Plexxikon Inc.: Employment. Zhang:Plexxikon Inc.: Employment. Ma:Plexxikon Inc: Employment. Narahara:Daiichi Sankyo Co, Ltd: Employment. Sumi:Daiichi Sankyo, Inc.: Employment. Bollag:Plexxikon Inc.: Employment. Levis:FUJIFILM: Consultancy, Research Funding; Daiichi Sankyo Inc: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Astellas: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Amgen: Consultancy, Honoraria; Menarini: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal