
Background: Quizartinib is a once-daily, oral, highly potent and selective FLT3 inhibitor with proven single-agent activity and a manageable safety profile in FLT3-ITD R/R AML. In the global, phase 3 QuANTUM-R study, quizartinib demonstrated a significant survival benefit over salvage chemotherapy(Cortes, et al. Lancet Oncol, 2019; NCT02039726). Additional support for the safety profile of quizartinib at the proposed dosing regimen (60 mg QD with a 30-mg starting dose) is provided by a pooled analysis of over 600 patients with R/R AML.
Methods: We pooled data from four studies: one phase 3 (QuANTUM-R), two phase 2, (ACC220-002 and 2689-CL-2004; Cortes, et al. Lancet Oncol, 2018; Cortes, et al. Blood, 2018), and one phase 1 (CP0001: only patients assigned to continuous daily dosing regimens are included in this analysis; Cortes, et al. J Clin Oncol, 2013) to provide an integrated safety summary of quizartinib monotherapy for the treatment of R/R AML. Treatment-emergent adverse events (TEAEs), on-treatment deaths, and adverse events of special interest (AESIs; QT prolongation and potential ventricular arrhythmias, hepatic disorders, and sequelae of cytopenias such as infections and bleeding) with quizartinib were analyzed.
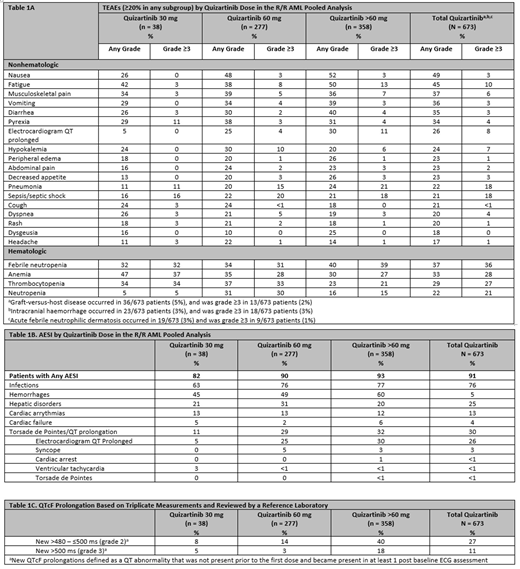
Results: The pooled data set included 673 patients; 51% were male, and median age was 59 years. Thirty-eight patients were assigned to quizartinib 30 mg, 277 to 60 mg, and 358 to >60 mg up to 300 mg. Overall, median treatment duration was 79 days (range, 1-1296 days) and was longest in the 60-mg dose group (95 days) vs the >60-mg (70.5 days) and 30-mg (66 days) groups. The overall TEAE profile was consistent with the results from the phase 3 QuANTUM-R study. Most on-treatment deaths (215 of 599 evaluable patients [36%]; defined as taking place between the first dose and ≤30 days after the last dose) were attributed to AML disease progression (130/599 [22%]), followed by TEAEs (83/599 [14%]), which were predominantly respiratory tract infections and events related to sepsis. TEAEs leading to discontinuation of quizartinib occurred in 173/673 patients (26%). In the 60-mg dose group (quizartinib proposed dosing regimen), the only TEAE associated with discontinuation in >2% of patients was pneumonia (6/277 patients [2%]). The most frequently reported grade ≥3 TEAEs were febrile neutropenia (240/673 [36%]), anemia (188/673 [28%]) and thrombocytopenia (182/673 [27%]) in the hematologic category and pneumonia (119/673 [18%]) and sepsis/septic shock (124/673 [18%]) in the nonhematologic category (Table 1A). In the 60-mg dose group, the most frequent AESI was infection (210/277 [76%]; Table 1B). Epistaxis and petechiae, mainly grade 1/2, were the most frequently reported hemorrhage TEAEs. TEAEs in the hemorrhage category occurred more frequently in the >60-mg dose group than the 60-mg dose group (215/358 [60%] and 136/277 [49%], respectively). The most frequent serious hemorrhage TEAEs were gastrointestinal hemorrhages (15/673 [2%]) and intracranial hemorrhage (10/673 [2%]). QTcF prolongation >500 ms (grade 3) occurred in 75/673 patients (11%), mostly in those receiving quizartinib >60 mg (n = 64) (Table 1C). In the 60-mg dose group, QTcF prolongation >500 ms occurred in 9/277 patients (3%). The median time to onset of QTcF prolongation >500 ms was shorter in the >60-mg dose group than in the 60-mg dose group (9 and 46 days, respectively). Arrhythmias potentially related to QTcF prolongation were infrequent; one event of torsade de pointes, and one event of fatal cardiac arrest (both at doses >60 mg) were observed. No dose effects were seen with hepatic disorders, cardiac arrhythmias, or cardiac failure AESI categories.
Conclusions: The overall safety profile of quizartinib in this R/R AML pooled analysis is consistent with that observed in QuANTUM-R. A reduction in the incidence of QTcF prolongation was noted with lower doses of quizartinib (30 or 60 mg vs >60 mg). There was also a reduced incidence of gastrointestinal symptoms, infections, and bleeding at lower doses of quizartinib (30 or 60 mg vs >60 mg). Results from this pooled analysis demonstrate that quizartinib is well tolerated at the proposed dosing regimen (60 mg QD with a 30-mg starting dose) in patients with R/R AML.
Cortes:BiolineRx: Consultancy; Biopath Holdings: Consultancy, Honoraria; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Merus: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Forma Therapeutics: Consultancy, Honoraria, Research Funding; Astellas Pharma: Consultancy, Honoraria, Research Funding; Jazz Pharmaceuticals: Consultancy, Research Funding; Sun Pharma: Research Funding; Immunogen: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding. Ganguly:Kite Pharma: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Seattle Genetics: Speakers Bureau; Daiichi Sankyo: Research Funding. Krämer:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Research Funding; BMS: Research Funding. Levis:FUJIFILM: Consultancy, Research Funding; Menarini: Consultancy, Honoraria; Novartis: Consultancy, Research Funding; Daiichi Sankyo Inc: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Astellas: Consultancy, Research Funding. Martinelli:Daiichi Sankyo: Consultancy, Honoraria; Amgen: Consultancy, Other: trial grant; Pfizer: Consultancy, Other: trial grant; Ariad: Consultancy, Other: trial grant; Incyte: Consultancy, Other: trial grant; Roche: Consultancy, Other: trial grant; Celgene: Consultancy, Honoraria, Other: trial grant; Janssen: Consultancy, Other: trial grant; Novartis: Consultancy, Other: trial grant; Abbvie: Consultancy, Honoraria, Other: trial grant. Perl:Takeda: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Daiichi Sankyo: Consultancy, Honoraria, Other, Research Funding; Astellas: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings as well as a medical writing company that assisted with manuscript preparation/submission and slide deck assembly for academic meeting presentations of trial data., Research Funding; Arog: Consultancy, Other: Non-financial support included travel costs for advisory board meetings.; AbbVie: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Actinium Pharmaceuticals: Consultancy, Honoraria, Other: Clinical Advisory Board member, Research Funding; Bayer: Research Funding; BioMed Valley Discoveries: Research Funding; FujiFilm: Research Funding; Novartis: Honoraria, Other: Advisory board, Non-financial support included travel costs for advisory board meetings as well as a medical writing company that assisted with manuscript preparation/submission and slide deck assembly for academic meeting presentations of the data., Research Funding; NewLink Genetics: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.; Agios: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Non-financial support included travel costs for advisory board meetings.; Jazz: Consultancy, Honoraria, Other: Non-financial support included travel costs for advisory board meetings.. Russell:Jazz: Consultancy, Honoraria, Speakers Bureau; DSI: Consultancy, Honoraria, Speakers Bureau; Astellas: Consultancy, Honoraria, Speakers Bureau; Pfizer Inc: Consultancy, Honoraria, Speakers Bureau. Choi:Daiichi Sankyo: Employment. Mendell:Daiichi Sankyo, Inc.: Employment. Namuyinga:Daiichi Sankyo: Employment. Pham:Daiichi Sankyo: Employment. Said:Daiichi Sankyo: Employment. Wang:Daiichi Sankyo: Employment. Mitov:Daiichi Sankyo UK Ltd: Employment. Kim:Daiichi Sankyo: Employment. Khaled:Alexion: Consultancy, Speakers Bureau; Daiichi Sankyo: Other: Travel support; Omeros: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal