
Background:
Older patients with acute myeloid leukemia (AML) have inferior outcomes when compared to younger patients. Hypomethylating agents (HMA) were established as the standard of care for patients who are unfit for intensive induction chemotherapy until HMA and venetoclax (HMA+ven) combination approval by the FDA in December 2018. Approval of HMA+ven was based on an early phase study which produced high response rates; however, the combination was not compared head-to-head with HMA alone. A randomized phase 3 study is currently underway. There is no data available comparing HMA+ven to HMA monotherapy in older patients (age ≥70 years), thus we aimed to characterize responses in older patients when treated with these two regimens.
Methods:
We retrospectively reviewed clinical and molecular data on 225 patients at Moffitt Cancer Center and Memorial Health System with newly diagnosed AML who were ≥ 70 years old and were treated with HMA monotherapy or HMA+ven combination. Clinical data was abstracted in accordance with institutional review board approved protocol. Patients were then divided in two subgroups: Cohort A) HMA monotherapy and B) HMA+ven combination. We calculated overall response rates (ORR) defined as patients achieving complete remission (CR), CR with incomplete hematologic recovery (CRi) or morphologic leukemia free state (MLFS). Fisher's Exact method was utilized to determine significance for categorical variables. All reported p-values are two sided. Next generation sequencing (NGS) results were analyzed using the TruSight Myeloid-54 gene panel with a sensitivity of 5%, and were characterized in patients treated in cohort B.
Results:
Among the 225 patients, 87% (n=196) were in cohort A and 13% (n=29) in cohort B. In cohort A, 36.7% were females compared to 27.6% in cohort B. Median age in both cohorts was 76 years (range: 70-90 years in cohort A) (range: 72-86 years in cohort B). Overall, 26% of the patients had adverse risk disease as defined by European Leukemia Net (ELN) classification in cohort A and 51.7% in cohort B. Baseline characteristics are described in Table 1.
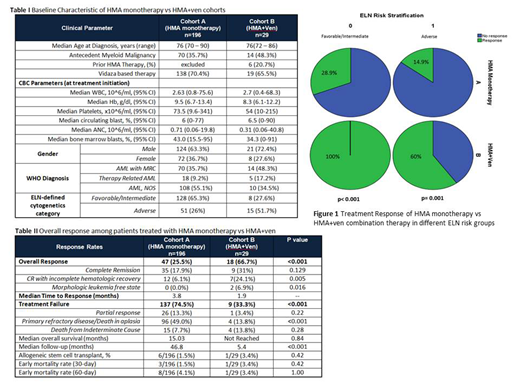
Overall response rate (ORR) of the entire cohort was 43.6% (n=92) (Table 2). ORR in cohort A was 25.5% (n=47) compared to 66.7% (n=18) in cohort B (p<0.001). The median time to response in cohort A was 3.8 mos and was 1.9 mos in cohort B. Looking only at the 66 patients with ELN-defined adverse risk, response data were available in 62 patients, and the ORR in both cohorts was 25.8% (n=16), and was significantly lower in cohort A compared to B (14.9% vs. 60%, respectively, p=0.001) (Figure 1). Among the 136 patients with favorable or intermediate risk disease, response data were available in 127 patients, and the ORR was 35.4% (n=45). In cohort A the ORR in favorable/intermediate patients was 28.9% (n=37), and in cohort B it was significantly higher at 100% (n=8) (p<0.001). Ten responding patients in cohort B had NGS data available at diagnosis and at the time of best response. Mutations cleared from the bone marrow in 60% (n=6) of these patients.
With a median follow up of 11.7 months, the median overall survival (mOS) of the entire cohort was 15.03 months. The median follow-up time in cohort A is 46 months and in cohort B is 5.4 months, making assessment of relapse free survival or overall survival in cohort B premature.
Early mortality rate was not different between the two cohorts (1.5% vs 3.4%, p=0.42).
Conclusion:
Our data provides convincing support that HMA+ven combination yields significantly higher response rates when compared to HMA monotherapy in newly diagnosed AML patients ≥70 years of age; an observation that is further strengthened by the short duration of follow-up in the HMA+Ven cohort. Responses are particularly striking in favorable and intermediate risk patients when treated with HMA+Ven. Overall our data supports the use of HMA+ven in the upfront setting for older patients with newly diagnosed AML. Additional follow-up in HMA+ven arm is needed to evaluate survival outcomes.
Kuykendall:Incyte: Honoraria, Speakers Bureau; Abbvie: Honoraria; Janssen: Consultancy; Celgene: Honoraria. List:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Lancet:Agios, Biopath, Biosight, Boehringer Inglheim, Celator, Celgene, Janssen, Jazz Pharmaceuticals, Karyopharm, Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Other: fees for non-CME/CE services . Komrokji:JAZZ: Speakers Bureau; Novartis: Speakers Bureau; JAZZ: Consultancy; Agios: Consultancy; Incyte: Consultancy; DSI: Consultancy; pfizer: Consultancy; celgene: Consultancy. Sallman:Celyad: Membership on an entity's Board of Directors or advisory committees. Talati:Celgene: Honoraria; Agios: Honoraria; Jazz Pharmaceuticals: Honoraria, Speakers Bureau; Daiichi-Sankyo: Honoraria; Astellas: Honoraria, Speakers Bureau; Pfizer: Honoraria. Sweet:Pfizer: Consultancy; Incyte: Research Funding; Jazz: Speakers Bureau; Stemline: Consultancy; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Celgene: Speakers Bureau; Agios: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal