
Background: Contemporary studies have identified >1 cycle of induction chemotherapy to induce complete remission (CR) as an independent risk factor for relapse and mortality in younger adults with acute myeloid leukemia (AML; Othus et al., Leukemia 2019). This study aimed at defining the potential impact of previous induction chemotherapy on the clinical benefit of relapse-preventive immunotherapy in AML.
Methods: In a previously reported phase 3 trial (Brune et al., Blood 2006; Martner et al., Blood Rev 2013), two hundred and sixty-one patients with AML in first CR (18-84 years, median 55) who were not eligible for allogeneic transplantation were randomly assigned to receive immunotherapy with histamine dihydrochloride and low-dose interleukin-2 (HDC/IL-2) or standard of care (no treatment, control). The HDC component of this regimen aims at countering immunosuppression induced by reactive oxygen species, which are formed by the NOX2 enzyme expressed by normal and malignant myeloid cells. HDC, a NOX2 inhibitor, improves the function and viability of anti-leukemic lymphocytes such as natural killer cells and cytotoxic T cells (Martner et al., J Pathol 2019). The immunotherapy was initiated in CR1 after the completion of chemotherapy with the aim to prevent relapse in the post-consolidation phase. Cycles 1 to 3 comprised 3 weeks on treatment and 3 weeks off treatment. In cycles 4 to 10 the off-treatment periods were extended to 6 weeks. In each cycle, patients in the treatment arm received HDC (Noventia Pharma, Milan, Italy) at 0.5 mg and human recombinant low-dose IL-2 (aldesleukin; 16 400 IU/kg; Chiron Corporation, Emeryville, CA) subcutaneously twice a day. All patients were followed for at least 36 months after random assignment. The median follow-up time was 48 months.
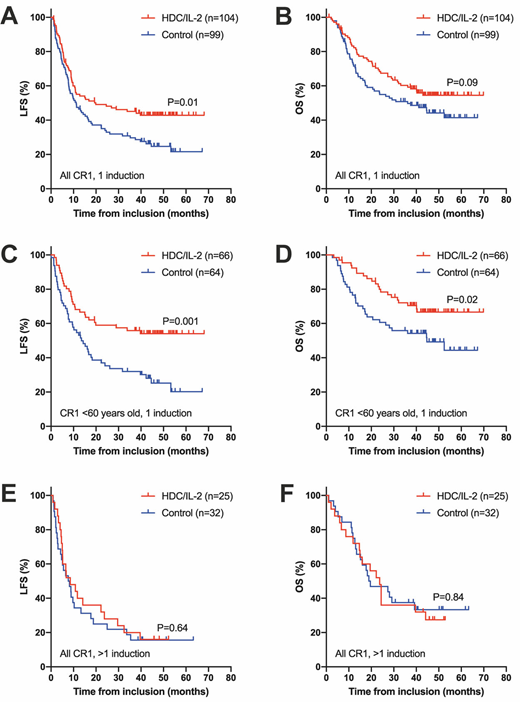
Results: The number of previous induction cycles was known in 260 patients, and 203 of these attained CR after 1 induction cycle (78%). Among the 57 remaining patients, forty-seven attained CR after 2 cycles, and 3 (n=7) or 4 (n=3) cycles were required to induce CR in 10 patients. We compared outcomes of patients who had achieved CR1 after 1 vs >1 cycle of induction chemotherapy and observed a non-significant trend towards inferior outcome in control arm patients who had required >1 induction with a similar trend in younger control patients (<60 years old). In the HDC/IL-2 arm, outcome was significantly superior in younger patients who had attained CR1 after 1 vs >1 induction (leukemia-free survival, LFS, P=0.0007, hazard ratio, HR, 1.67-6.77; overall survival, OS, P=0.009, HR 1.30-6.33 in multivariable analyses correcting for potential confounding factors for relapse and death).
When comparing outcomes between treatment and control arms within groups of patients who had attained CR1 after 1 cycle of induction chemotherapy, we observed that the LFS of patients in the HDC/IL-2 arm was significantly superior over controls (P=0.01 in multivariable analysis, N=203). In patients who were <60 years old at random assignment (N=130), treatment with HDC/IL-2 entailed significantly improved LFS (P=0.0008 vs control, HR 0.29-0.72) and OS (P=0.01, HR 0.28-0.75). HDC/IL-2 did not improve LFS or OS in patients who had required >1 cycle of induction to attain CR1 (P>0.5 vs control) and was not significantly beneficial in older patients. A test of interaction supported that the effect size of HDC/IL-2 vs control for LFS was more pronounced in younger patients who had attained CR after 1 (HR=0.48) vs >1 (HR=1.33) cycle of induction (P=0.04). Figure 1 shows Kaplan-Meier analyses of outcome, with unadjusted P-values from logrank tests, in the phase 3 trial arms among all patients attaining CR1 after 1 induction (A and B), in corresponding patients <60 years old (C and D) and in all patients who had attained CR1 after >1 cycle of induction (E and F).
Conclusions: These findings imply that the efficiency of previous induction chemotherapy independently determines the clinical benefit of relapse-preventive immunotherapy in adult AML patients who are not candidates for allogeneic transplantation. The results may have implications for the selection of patients who are likely to benefit from treatment with HDC/IL-2 and for the design of clinical trials that evaluate relapse-preventive immunotherapy.
Thorén:Cytovia, New York: Patents & Royalties: Holds patents protecting the use of NOX2 inhibitors in cancer. Martner:Cytovia, New York: Patents & Royalties: Holds patents protecting the use of NOX2 inhibitors in cancer. Hellstrand:Cytovia, New York: Patents & Royalties: Holds patents protecting the use of NOX2 inhibitors in cancer.
HDC/IL-2 is approved for the prevention of AML relapse within the EU but is not yet approved in the US.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal