Introduction:
Relapse of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) after allogeneic stem cell transplantation (ASCT) is the major cause of mortality. One of the main risk factors for this outcome is the persistence of measurable residual disease (MRD) after achieving a remission and prior to ASCT conditioning. Relapse after ASCT occurs due to persistence of the leukemic stem cell (LSCs) population; LSCs have aberrant activation of the Hedgehog signaling pathway, and targeting this pathway has been shown to eradicate LSCs. Therefore, interruption of Hedgehog signaling might impact LSCs and prevent relapse after ASCT. Glasdegib is an inhibitor of Smoothened, a key mediator of the Hedgehog signaling pathway, and is approved for newly diagnosed AML patients. We hypothesized that targeting Smoothened with glasdegib in the post-ASCT setting for patients at high risk for relapse would improve relapse free survival (RFS).
Methods:
This was a multi-institution phase 2 pilot clinical trial that enrolled patients between January 2014 and July 2018. Patients were ≥18 years, had AML or MDS, ECOG performance status ≤ 2 and preserved organ function. All patients were enrolled prior to transplant, and were deemed high-risk for post ASCT relapse, defined by one of the following: A)The presence of MRD after remission and prior to conditioning, as measured by multi-parameter flow cytometry (MPFC, Hematologics); B)The persistence of cytogenetic abnormalities; or C)For non-myeloablative transplants, a non-myeloablative transplant relapse risk score >0 (calculated based on 2nd (+1) vs 3rd (+2) complete remission, unfavorable cytogenetics (+1), absence of pre-ASCT peripheral blood count recovery (+0.5), and >18 months from diagnosis to ASCT (+0.5)). 100 mg of glasdegib was given daily starting between days 28-50 post ASCT, after stable engraftment was achieved. Patients were treated continuously for up to 12 months, or until toxicity or relapse. The primary endpoint was one-year RFS. Secondary endpoints included incidence and severity of adverse events (AEs), and one-year overall survival (OS).
Results:
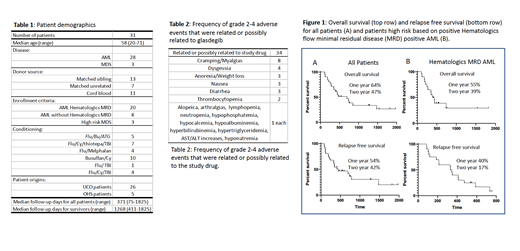
31 patients were enrolled; 27 had AML and 4 had MDS. Median age was 58 years (20-71). 20/27 AML patients were deemed high risk by the presence of MRD by MPFC pre-transplant (Table 1). There were 85 AEs deemed related or possibly related to glasdegib and of these 34 were ≥grade 2 (Table 2). The most common related/possibly related ≥grade 2 AEs were cramping/myalgias (23.5%), dysgeusia (11.8%) and anorexia/weight loss, nausea, and diarrhea (all 8.8%). There were no related grade 5 AEs. 19 patients had dose interruptions of glasdegib and 5 were dose-reduced due to related/possibly related AEs. Two patients discontinued glasdegib permanently due to AEs (myalgias and ALT/AST increases, respectively). With median follow up time of 371 days, 1-year and 2-year RFS was 54% and 32%, respectively. 1-year and 2-year OS were 64% and 37%, respectively. Analyzing only AML patients who were high-risk for relapse by MPFC, the 1-year and 2-year RFS were 40% and 17%, respectively, and 1-year and 2-year OFS were 55% and 39%, respectively (Figure 1). One and two year cumulative incidence of treatment related mortality (TRM) for all patients was 10% and 14% respectively, and for AML high-risk by MPFC, 10% and 10% respectively. One and two year cumulative incidence of relapse for all patients was 38% and 53% respectively, and for AML high-risk by MPFC, 50% and 73% respectively.
Conclusions:
Previous studies have demonstrated a 1-year RFS of approximately 30% for patients at high risk for post-ASCT relapse as defined by the presence of MRD by MPFC. Using this measure, single-agent glasdegib did not appear to significantly reduce post-transplant relapse for AML and MDS patients at high risk for relapse. Most AEs were not high grade but impacted quality of life; the majority of patients required drug holds, and among patients not experiencing relapse or treatment-related mortality, most required dose reductions and two withdrew due to toxicity. It is imperative to identify well tolerated post-ASCT interventions that can prevent relapse in patients who have risk factors for this outcome.
Vasu:Boehringer Ingelheim: Other: Travel support; Seattle Genetics: Other: Clinical trial support. Devine:Bristol Myers: Other: Grant for monitoring support & travel support; Magenta Therapeutics: Other: Travel support for advisory board; My employer (National Marrow Donor Program) has equity interest in Magenta; Kiadis Pharma: Other: Protocol development (via institution). Pollyea:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Diachii Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Forty-Seven: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celyad: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Glasdegib in the post-transplant maintenance setting
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal