Introduction: Mosunetuzumab (M; RG7828) is a full-length, fully humanized immunoglobulin G1 (IgG1) bispecific antibody targeting both CD3 (on the surface of T cells) and CD20 (on the surface of B cells). Clinical data from GO29781 (NCT02500407), a Phase I/Ib study in relapsed or refractory (R/R) non-Hodgkin lymphoma (NHL) pts, indicate that M has promising efficacy and safety (Budde et al. ASH 2018; Bartlett et al. ASCO 2019). We report the characterization of exposure-response (E-R) relationships for safety and efficacy to inform clinical dose/regimen finding.
Methods: DATA: Data from Group A (0.05 to 2.8mg q3w dosing) and Group B (0.4/1/2.8 to 1/2/27mg Cycle 1 Day 1/8/15 step-up dosing, followed by q3w dosing) were analyzed. E-R for safety was assessed in 142 evaluable pts by dosing Group. E-R for efficacy was assessed in 130 evaluable pts by aggressive (a) (n=83, primarily DLBCL and transformed FL) and indolent (i) (n=47, primarily FL) NHL histologies. PHARMACOKINETIC (PK) ENDPOINTS: Pt-specific PK exposure metrics, including AUC and Cmax, were derived using a preliminary two-compartment population PK model with time-dependent clearance. Due to the presence of residual rituximab (R) at baseline from prior treatments, an additional exposure metric of average CD20 receptor occupancy (RO%) of M was derived based on the serum PK and binding affinity (KD) of both agents to assess the impact of target binding competition over time. E-R ANALYSES: Objective response rate (ORR), complete response rate (CRR), and adverse event (AE) rates, including cytokine release syndrome (CRS) and neurological AEs (NAE), were summarized by exposure-tertiles and the E-R relationships modeled by logistic regression (linear or Emax models). Response rates and E-R were further assessed in subgroups by RO% tertiles and baseline factors, such as the number of prior lines of therapy, refractory status, lactate dehydrogenase level, and tumor burden, and modeled by multivariate logistic regression.
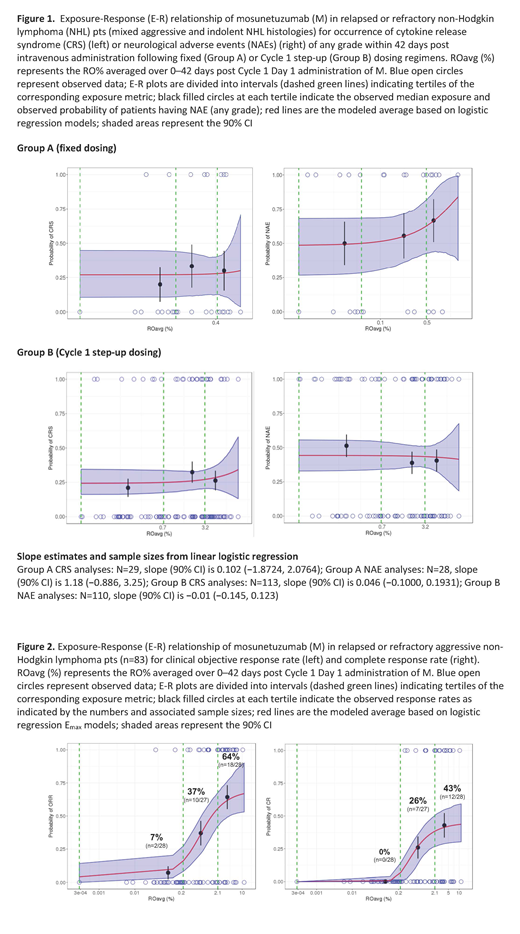
Results: R was present at modest levels at baseline in aNHL pts (median, 3 µg/ml, 33% of pts below quantitation limit [BQL] of 0.5µg/ml) but minimally in iNHL pts (median, <BQL). SAFETY: No statistically significant E-R relationships were found, although a visual trend of E-R for NAE was observed after fixed dosing, but not step-up dosing (Figure 1). EFFICACY: In aNHL pts, E-R analysis indicated that RO%, rather than Cmax or AUC, was strongly correlated with clinical response (Figure 2). The relevance of RO% was supported by strong correlations with pharmacodynamic biomarkers, such as T-cell activation and IFN-γ (Hernandez et al. ASH 2019). Pt baseline factors were balanced across RO% tertiles, except for time since last R dose, as expected per definition of RO%. Maximal clinical responses were achieved with greater RO%, reflected by the E-R plateau and described by an Emax logistic regression model. The observed ORR and CRR among pts in the top RO% tertile was 64% (18/28 pts) and 43% (12/28), respectively (Figure 2), denoting favorable therapeutic potential of M in R/R aNHL. The E-R relationship was statistically significant on top of evaluated baseline pt factors, reflected by positive E-R trends in all pt subgroups. Clinical responses were observed at 20mg even in the presence of high residual R levels (20-40µg/ml) in pts who had recently received R (within 3 months). Further dose escalation is expected to enhance efficacy by increasing the proportion of pts achieving sufficient target engagement. For iNHL pts, AUC correlated well with ORR and CRR, with no clinically meaningful interference from R due to the indolent nature of the disease. The observed ORR and CRR in pts in the top AUC tertile was 75% (12/26 pts) and 44% (7/16), respectively. M was active at dose levels (1.2-27 mg) that translated to a RO% of 0.2-10% (far below receptor saturation), consistent with its mechanism as an agonist of T-cell activation.
Conclusions: E-R analyses indicate promising therapeutic benefit/risk of M in R/R NHL pts. Step-up dosing mitigated CRS-related toxicities, resulting in a broad therapeutic window of M that enables further dose escalation to maximize efficacy. The lack of E-R for safety was contrasted by a steep E-R for efficacy whereby higher M exposures overcome target binding competition in pts with residual R. Our analyses provide useful guidance for the dosing strategy of CD20-targeting T-cell bispecifics.
Li:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Bender:Genentech, Inc.: Employment, Equity Ownership. Yin:Genentech, Inc: Employment, Equity Ownership. Li:Pro Unlimited - Genentech, Inc. contractor: Employment; F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Zhang:Genentech, Inc., SSF: Employment. Hernandez:Genentech, Inc.: Employment, Equity Ownership. Kwan:Genentech, Inc: Employment, Equity Ownership. Sun:Harpoon Therapeutics: Employment, Equity Ownership; F. Hoffmann-La Roche Ltd / Genentech, Inc.: Employment. Adamkewicz:Genentech, Inc.: Employment; F. Hoffmann-La Roche Ltd: Equity Ownership. Wang:Genentech, Inc.: Employment; F. Hoffmann-La Roche Ltd: Equity Ownership. Dimier:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Lim:Genentech, Inc.: Employment. O'Hear:F. Hoffmann-La Roche Ltd: Equity Ownership; Genentech, Inc.: Employment. Sehn:Merck: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Janssen-Ortho: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; TEVA Pharmaceuticals Industries: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Apobiologix: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria; Astra Zeneca: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; F. Hoffmann-La Roche/Genentech: Consultancy, Honoraria, Research Funding; Verastem: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen-Ortho: Honoraria; Lundbeck: Consultancy, Honoraria; Acerta: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Merck: Consultancy, Honoraria. Flinn:AbbVie, Seattle Genetics, TG Therapeutics, Verastem: Consultancy; TG Therapeutics, Trillum Therapeutics, Abbvie, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, Verastem, Gilead Sciences, Astra Zeneca (AZ), Juno Therapeutics, UnumTherapeutics, MorphoSys, AG: Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Acerta Pharma, Agios, Calithera Biosciences, Celgene, Constellation Pharmaceuticals, Genentech, Gilead Sciences, Incyte, Infinity Pharmaceuticals, Janssen, Karyopharm Therapeutics, Kite Pharma, Novartis, Pharmacyclics, Portola Pharmaceuticals: Research Funding; TG Therapeutics, Trillum Therapeutics, Abbvie, ArQule, BeiGene, Curis, FORMA Therapeutics, Forty Seven, Merck, Pfizer, Takeda, Teva, Verastem, Gilead Sciences, Astra Zeneca (AZ), Juno Therapeutics, UnumTherapeutics, MorphoSys, AG: Research Funding. Yoon:Novartis: Consultancy, Honoraria; Janssen: Consultancy; Kyowa Hako Kirin: Research Funding; Amgen: Consultancy, Honoraria; Yuhan Pharma: Research Funding; Genentech, Inc.: Research Funding; MSD: Consultancy. Budde:F. Hoffmann-La Roche Ltd: Consultancy. Li:Genentech, Inc. / F. Hoffmann-La Roche Ltd: Employment. Wei:Genentech, Inc./F. Hoffmann-La Roche Ltd: Employment, Equity Ownership.
Mosunetuzumab (RG7828) is a full-length, fully humanized immunoglobulin G1 (IgG1) bispecific antibody targeting both CD3 (on the surface of T cells) and CD20 (on the surface of B cells). Mosunetuzumab redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab is an investigational agent.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal