Introduction: FL, the most common indolent non-Hodgkin lymphoma, is characterized by a defective immune microenvironment that suppresses normal T-cell and natural-killer (NK)-cell activity. The clinical course is often depicted by high initial response rates coupled with a prolonged natural history and repeated relapses with most patients (pts) succumbing to their disease. Effective, well tolerated therapies are desirable. Obinutuzumab (O) is a humanized, type II anti-CD20 monoclonal antibody glycoengineered for enhanced antibody-dependent cellular cytotoxicity (ADCC). Lenalidomide (len) is an immunomodulatory agent that binds the cereblon E3 ubiquitin ligase complex resulting in recruitment, ubiquitination, and degradation of transcription factors Aiolos and Ikaros resulting in T-cell and NK-cell activation. Therefore, combining O with len is anticipated to be synergistic in augmenting the innate and adaptive immune response in FL. The combination has been shown to be well tolerated and effective in relapsed FL (Fowler ICML 2017). Therefore, we sought to explore the efficacy and safety of O-len in previously untreated, high tumor burden FL.
Methods: We conducted as single-center, phase 2 study in previously untreated, stage II, III, or IV, high tumor burden (defined by GELF) FL (grade 1, 2 or 3A). Pts received 1000mg of O on days 1, 8, and 15 of cycle 1, day 1 of cycles 2-6, and day 1 of even numbered cycles, cycle 8-30. Cycle length was 28 days. Len was administered as 20mg on days 1-21 of cycles 1-6. Pts in a complete response (CR) after 6 cycles received reduced dose len (10mg on days 1-21) for cycles 7-18. Among pts in a partial response (PR) after 6 cycles, len was continued at 20mg for the next 3-6 cycles or until CR, whichever occurred first, len was then dose reduced to 10mg on days 1-21 for the remainder of 18 cycles. The primary endpoint was progression-free survival (PFS) at 2 years (according to Lugano 2014 criteria). Secondary endpoints included: safety, CR, PR, overall response (ORR), and overall survival (OS).
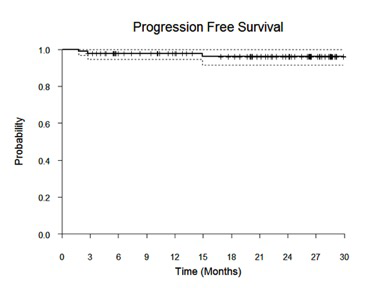
Results: 90 pts with high tumor burden FL were enrolled. Median age was 58 years (range 33-84), 52% (N=47) were male, 67 (74%) had an ECOG performance status of 0, 9 (10%) had stage II, 23 (26%) stage III, and 58 (64%) had stage IV disease. The majority had grade 1/2 FL (80%). Twenty-one percent had low risk FLIPI scores, 37% intermediate risk, and 42% were high risk. With a median follow-up of 22 months (range 1-30 months), the 2-year PFS estimate is 96% (95% CI 92-100%) with only 2 pts experiencing progression to date. The ORR is 98% (85 CR, 1 PR), 92% achieved a CR at the first response assessment (cycle 4, day 1). Correlative studies are underway including serial circulating tumor DNA measurements.
No deaths have been observed to date. Eleven pts (12%) discontinued therapy as a result of an adverse event (AE), upper respiratory infection was the most common reason (N=5). Other reasons included bradycardia with sick sinus syndrome, urinary tract infection, constipation, abdominal pain, fatigue, foot neuroma (N=1 for each instance). The most common grade 3 or higher AEs include neutropenia (16%, grade 3 N=5, grade 4 N= 9), rash (10%), lung infection (4%), neutropenic fever (1%).
Conclusions: O-Len was associated with very high CR rates and 2-year PFS estimates in untreated, high tumor burden FL. The toxicity profile was manageable. Further study of this effective, immune therapy approach in untreated FL is warranted.
Nastoupil:Bayer: Honoraria; Celgene: Honoraria, Research Funding; Genentech, Inc.: Honoraria, Research Funding; Gilead: Honoraria; Janssen: Honoraria, Research Funding; Novartis: Honoraria; TG Therapeutics: Honoraria, Research Funding; Spectrum: Honoraria. Westin:Genentech: Other: Advisory Board, Research Funding; Unum: Research Funding; Novartis: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board, Research Funding; Juno: Other: Advisory Board; 47 Inc: Research Funding; MorphoSys: Other: Advisory Board; Kite: Other: Advisory Board, Research Funding; Curis: Other: Advisory Board, Research Funding; Celgene: Other: Advisory Board, Research Funding. Parmar:Cellenkos Inc.: Equity Ownership, Membership on an entity's Board of Directors or advisory committees, Research Funding. Wang:Pharmacyclics: Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; Acerta Pharma: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; MoreHealth: Consultancy, Equity Ownership; Kite Pharma: Consultancy, Research Funding; Guidepoint Global: Consultancy; BioInvent: Consultancy, Research Funding; VelosBio: Research Funding; Loxo Oncology: Research Funding; Celgene: Honoraria, Research Funding; Juno Therapeutics: Research Funding; Aviara: Research Funding; Dava Oncology: Honoraria. Neelapu:Acerta: Research Funding; Celgene: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; Allogene: Consultancy; Cell Medica: Consultancy; Unum Therapeutics: Consultancy, Research Funding; Pfizer: Consultancy; Poseida: Research Funding; Karus: Research Funding; Novartis: Consultancy; Incyte: Consultancy; BMS: Research Funding; Cellectis: Research Funding; Precision Biosciences: Consultancy; Merck: Consultancy, Research Funding. Fowler:ABBVIE: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis Pharmaceuticals Corporation: Consultancy.
Lenalidomide in untreated follicular lymphoma
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal