Epigenetic regulators in normal and malignant hematopoiesis have been shown to be important in normal and malignant stem cell self-renewal function and myeloid leukemogenesis. While epigenetic dysregulation can occur through activating and/or loss-of-function mutations, these regulators can also be modulated by other regulators, such as microRNAs. Specifically, miR-29 has been previously identified as an "epi-mir" for contributing to epigenetic regulation by altering expression of DNMT3a and TET. We and others have previously shown that in the hematopoietic system, miR-29 is a positive regulator of hematopoietic stem cell (HSC) self-renewal, is upregulated in AML blasts, and when over-expressed in transplanted HSCs and immature progenitors, leads to a myeloproliferative-like disorder that progresses to acute myeloid leukemia (AML).
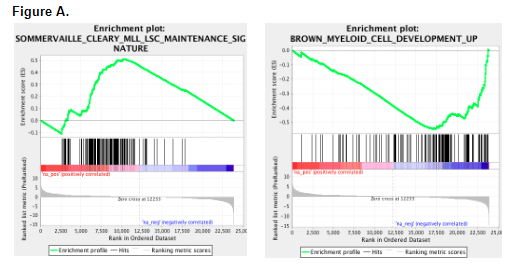
To investigate how miR-29 shapes the leukemic stem cell (LSC) epigenome, we transduced the MLL-AF9 fusion oncogene into WT and mir29a/b1 null Lin-ckit+sca1+ (LSK) cells and transplanted them into recipient mice. Transplantation of MLL-AF9+ miR-29 null cells into lethally irradiated recipients resulted in an increased disease latency compared to recipients of WT MLL-AF9+ cells (median: 56 vs 151 days, p<0.001). Characterization of miR-29 null blasts revealed increased expression of myeloid differentiation markers (CD11b, CD14), increased apoptosis, and reduced CFU capacity, consistent with decreased LSC self-renewal. To gain molecular insights into this phenotype, we interrogated RNA-seq data generated from WT and miR-29 null blasts. Compared to WT blasts, miR-29 null blasts showed loss of LSC gene signatures and enrichment for myeloid lineage genes, consistent with increased differentiation (Figure A). In addition, GSEA showed enrichment of H3K27me3, H3K4me2, and gene-specific polycomb group (PcG) associated pathways. On further validation, ChIP-Seq showed overall genome-wide reduction of DNA modification markers such as H3K27me3, H3K9me3, H3K36me3, H3K79me2, and H3K4me3 in miR-29 null blasts compared to WT. Hence, we hypothesized that miR-29 likely targets epigenetic regulators critical for LSC maintenance, and that loss of miR-29 rewires the LSC epigenetic landscape to induce differentiation and abrogate LSC self-renewal. In order to identify downstream mediators of the miR-29 null blast epigenetic phenotype, we used an shRNA library against 500 known epigenetic regulators. The top enriched genes in miR-29 null blasts were members of the PcG family, and as predicted, mRNAs encoding these proteins were upregulated in miR-29 null blasts, including CBX2. These findings were further corroborated by comparing the transcriptomes of AML patient samples to normal hematopoietic stem/progenitor cells (https://gexc.riken.jp), which showed that CBX2 has lower expression in LSCs and blasts compared to normal HSCs and GMPs. When we overexpressed CBX2 in K562 and THP-1 myeloid leukemia cell lines, we observed increased apoptosis and myeloid differentiation as well as impaired clonogenic capacity in vitro, suggesting that downregulation of CBX2 is important in myeloid leukemogenesis. Collectively, our studies indicate that miR-29 promotes LSC function by restricting CBX2 expression to maintain the AML epigenetic landscape.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal