Background: Follicular lymphoma (FL) is the most frequent indolent lymphoma. While immunochemotherapy (IC) treated FL patients who achieve event-free status at 24 months after diagnosis (EFS24) have the subsequent life expectancy of the general population, those who fail to achieve EFS24 have aggressive disease with poor outcomes. Similarly, in FL patients initially observed or treated with rituximab monotherapy, EFS at 12 months (EFS12) is a strong predictor of subsequent outcome. Thus, an unmet patient need is to predict at diagnosis those at greatest risk of early failure in order to identify better treatments. The lymphoma microenvironment may be a key determinant of early failure. Therefore, we aimed to improve risk stratification of newly diagnosed FL patients by using a discovery and validation study design to identify microenvironment determinants of early failure and then integrate them into the Follicular Lymphoma International Prognostic Index (FLIPI).
Patients and Methods: We evaluated 496 patients with FL grade 1-3A who were prospectively enrolled into the Molecular Epidemiology Resource (MER) cohort from 2002-2012. In the discovery set of patients (N=166), we stained tissue microarrays for CD4, CD8, FOXP3, CD32b, CD14, CD68, CD70, SIRPa, TIM3, PD-1 and PDL1. Immunohistochemical staining was determined both within and between malignant follicles. Stains were scored to the nearest decile as the percentages of cells positive. Scores for each stain were dichotomized as 0% vs >0% except for CD32b (<20% vs ≥20%), CD70 (<10% vs ≥10%), and SIRPa (<30% vs ≥30%). EFS was defined as time from diagnosis to progression, relapse, retreatment, or death. Early failure was defined as failing to achieve EFS24 for patients treated with IC at diagnosis and failing to achieve EFS12 for patients who were observed or received other treatments. Risk of early failure was estimated using odds ratios (ORs) and 95% confidence intervals (CI) from logistic regression models. Markers at P≤0.15 were brought forward for validation in a separate set of patients from the MER cohort (N=330), with P<0.005 being declared statistically significant after a Bonferroni correction (0.05/10 tests). In the combined dataset, we also used Cox regression to assess associations with continuous EFS and overall survival (OS). Mass cytometry (CyTOF) was then performed on a subset of patients (N=51) to comprehensively characterize the phenotype of intratumoral immune cells associated with outcome.
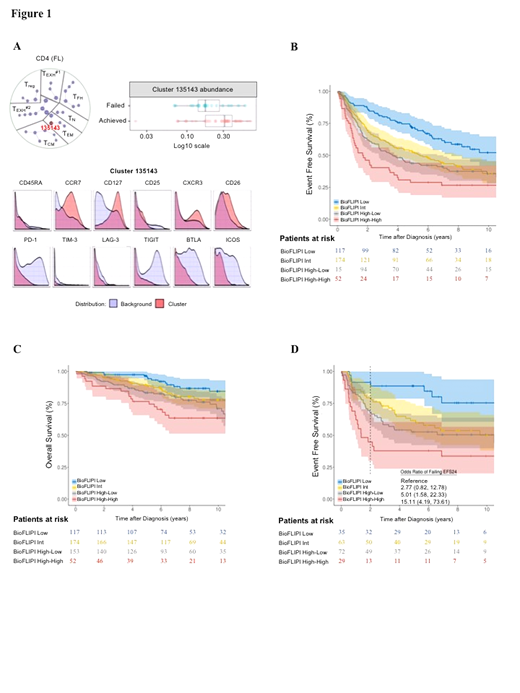
Results: In the discovery set, CD4, CD8, FOXP3, PD-1, and SIRPα were associated with risk of early failure at P≤0.15 and were subsequently evaluated in the validation set. Only CD4 expression inside the follicle validated. Specifically, lack of CD4 expression inside the follicle was associated with higher risk of early failure in the discovery (OR=1.77, P=0.15) and validation (OR=2.59, P=0.001) sets, with a pooled OR=2.29 (95% CI 1.47-3.58; P<0.001). CyTOF analysis characterized the favorable CD4+ T-cell population associated with achieving EFS12/24 as activated effector memory T cells lacking exhaustion markers (Figure 1A). Adjustment of CD4 expression for FLIPI in the combined dataset did not weaken this association (OR=2.38, 95% CI 1.51-3.79), and lack of CD4 expression was prognostic within each FLIPI group 0-1 (OR=2.65; p=0.036), 2 (OR=2.07; P=0.064) and 3-5 (OR=2.51; P=0.015). We next established a bio-clinical risk model (termed BioFLIPI) that combined CD4 intrafollicular expression and FLIPI into a 1-4 scale, where any expression of intrafollicular CD4 moved a patient up one FLIPI risk group, adding a fourth (high) risk group for FLIPI 3-5 and any CD4 inside expression. The BioFLIPI score was strongly associated with risk of early failure: compared to a BioFLIPI score of 1 (24% of patients), patients with a score of 2 (35% of patients; OR=2.17, 95% CI 1.08-4.69), 3 (31% of patients; OR=3.53, 95% CI 1.78-7.54), and 4 (10% of patients; OR=8.92, 95% CI 4.00-21.1) had increasing risk of early failure. The c-statistic for BioFLIPI was 0.665, which was higher than that for FLIPI alone (0.636). BioFLIPI was also strongly predictive of continuous EFS [Figure 1B], OS [Figure 1C], and for EFS and EFS24 when restricted to IC-treated patients [Figure 1D].
Conclusion: Integration of CD4 expression inside the follicle at diagnosis with FLIPI improves identification of FL patients at risk for early failure.
Novak:Celgene Coorperation: Research Funding. Nowakowski:Curis: Research Funding; Bayer: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; F. Hoffmann-La Roche Ltd: Research Funding; Selvita: Membership on an entity's Board of Directors or advisory committees; NanoString: Research Funding; Genentech, Inc.: Research Funding; MorphoSys: Consultancy, Research Funding. Cerhan:NanoString: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding. Ansell:LAM Therapeutics: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Affimed: Research Funding; Trillium: Research Funding; Bristol-Myers Squibb: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Trillium: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; Trillium: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; LAM Therapeutics: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; LAM Therapeutics: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; LAM Therapeutics: Research Funding; Trillium: Research Funding; LAM Therapeutics: Research Funding; Affimed: Research Funding; Regeneron: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; Regeneron: Research Funding; Seattle Genetics: Research Funding; Regeneron: Research Funding; Bristol-Myers Squibb: Research Funding; Trillium: Research Funding; Regeneron: Research Funding; Mayo Clinic Rochester: Employment; Affimed: Research Funding; Mayo Clinic Rochester: Employment; Seattle Genetics: Research Funding; Affimed: Research Funding; Bristol-Myers Squibb: Research Funding; Bristol-Myers Squibb: Research Funding; Affimed: Research Funding; Trillium: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Trillium: Research Funding; Mayo Clinic Rochester: Employment; Regeneron: Research Funding; Seattle Genetics: Research Funding; Mayo Clinic Rochester: Employment; Mayo Clinic Rochester: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal