Introduction:
There is a growing shortage of platelets, the principal blood cells responsible for clot formation and blood vessel repair at sites of active bleeding. Platelet BioGenesis (PBG) is developing a commercial-scale, donor-free platelet (PLT+) production process using a cGMP-grade human induced pluripotent stem cell line (hiPSC) to address this issue. The PLT+ activity is being characterized extensively through multiple approaches that measure hemostatic function both in vitro and in vivo, with the ultimate goal of determining a clinical dose in humans. Alongside tests such as the current gold standard thrombin generation assay (TGA), we have developed an in-house microfluidic model to measure the ability of PLT+ to adhere to extracellular proteins under flow. The assay captures the surface receptor dynamics, as well as the ability of PLT+ to initiate coagulation.
Materials and Methods:
The thrombin generation assays are performed according to manufacturer's instructions. For the in-house microfluidic assay (MFA), fibrillar collagen, fibrinogen, or von Willebrand Factor is patterned in a commercial microfluidic device (Ibidi Slide VI 0.1). PLT+ (DNA-, calcein +, CD61+, CD42a+ cells from our bioreactor) are concentrated in normal pooled plasma, platelet additive solution, or platelet wash buffer to a normalized concentration of 1e8/mL, and are labeled with the mitochondrial dye DiOC6. Calcium chloride (7.5 mM) is added and the solution is perfused with a syringe pump at 50 1/s over the bioactive surface for 10 min. Images are captured every 10 seconds and quantified with ImageJ. After perfusion, the samples are fixed with 4% paraformaldehyde and can be stained for further characterization.
Results and Discussion:
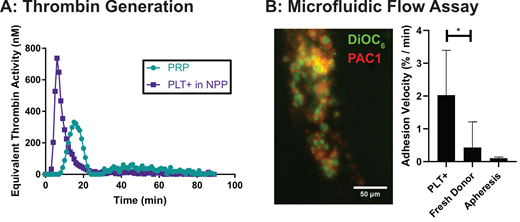
PLTs+ are functionally non-inferior to human donor (blood) platelets and appear more active than Day 4/5 stored apheresis unit platelets. The TGA of PLT+ shows a more rapid generation of thrombin but similar total potential to donor platelets (Figure A). In the microfluidic assay, PLT+ (green DiOC6, red PAC1, figure B) adhere readily to the collagen surface under low shear flow, but do not adhere to non-functionalized surfaces, indicating GPVI functionality. As measured by surface coverage, the PLT+ resuspended in PAS adhere faster to the surface than either freshly washed donor platelets or apheresis platelets (p=0.01). When the PLT+ are preincubated in normal pooled plasma prior to perfusion, we observe fibrin formation and the aggregates show clot retraction, which supports the TGA results. Adhesion to surface bound fibrinogen and VWF is also comparable to donor platelets (GP IIb/IIa and GPIb function,). Combined, the TGA and MFA data reflect results of traditional more resource intensive assays, suggesting their viability as a rapid and flexible platform to study platelet function and function as release assays for in vitro generated PLTs+
Conclusion:
Current work demonstrates that PLT+ are functional and a future alternative to donor derived platelet units. The MFA is a valuable tool for the characterization of this new therapeutic because of its specific control of surface protein-receptor interactions, shear forces, and flexibility to look at multiple markers. Alongside the TGA, the MFA will be a release assay for the PLT+.
Lehmann:Platelet Biogenesis: Employment. Burton:Platelet Biogenesis: Employment. Szemethy:Platelet Biogenesis: Employment. Valdez:Platelet BioGenesis: Employment. Thon:Platelet BioGenesis: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal