Introduction Concizumab is an anti-tissue factor pathway inhibitor (TFPI) monoclonal antibody in clinical development for the subcutaneous prophylactic treatment of hemophilia patients. We present results from the main part (at least 24 weeks) of the concizumab explorer4 phase 2 trial (NCT03196284) in hemophilia A/B with inhibitor (HAwI/HBwI) patients.
Methods The primary objective was to assess the efficacy of once-daily subcutaneous concizumab in preventing bleeds in HAwI/HBwI patients. Secondary objectives were the assessment of safety, including concomitant use of recombinant activated factor VII (rFVIIa), and immunogenicity. Patients were randomized 2:1 to concizumab prophylaxis or rFVIIa on-demand treatment via an interactive web-response system. A concizumab loading dose (0.5 mg/kg) was administered, followed by 0.15 mg/kg daily with potential dose escalation to 0.20 and 0.25 mg/kg. Efficacy was evaluated as the number of bleeding episodes (annualized bleeding rate [ABR]) at last dose level. The number of adverse events (AEs) and the occurrence of anti-drug antibodies (ADAs), as well as coagulation-related parameters were evaluated. Concizumab and free TFPI plasma levels were measured by ELISA, and peak thrombin generation (TG) potential using a standardized assay.
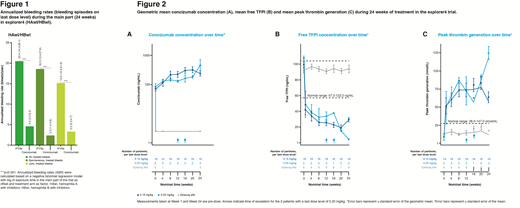
Results 26 patients were randomized; 9 HAwI and 8 HBwI patients were exposed to concizumab, and 9 patients to rFVIIa (7 with HAwI and 2 with HBwI). All 25 patients who completed the main 24-week part of the trial chose to continue to the extension part. The estimated ABR at the last dose level for concizumab prophylaxis was 4.5 (95% CI: 3.2−6.4) and for rFVIIa on demand, 20.4 [95% CI: 14.4−29.1] (Figure 1). There was a 78, 88 and 79% reduction in all treated bleeds and in spontaneous and joint bleeds, respectively, with concizumab prophylaxis compared with on-demand treatment (Figure 1). Concizumab concentration varied considerably between patients on the same dose level. Increasing concizumab dose was associated with lower free TFPI and normalized TG potential (Figure 2). No deaths, thromboembolic events or AE-related withdrawals occurred. No safety concerns with concomitant use of concizumab and rFVIIa were identified. Three patients had positive (very-low to medium-titer) ADA tests (titer range: 1 to 128), but with no apparent clinical effect. As expected, elevated prothrombin fragment 1+2 and D-dimers were observed across all concizumab dose levels, reflecting the hemostatic effect of concizumab.
Conclusions In the phase 2 explorer4 trial, concizumab was efficacious and safe as a subcutaneous prophylactic treatment in HAwI patients, as well as in HBwI patients for whom there is currently no prophylactic regimen available. There was no difference in safety and efficacy across hemophilia subtypes, including with the concomitant use of concizumab and the bypassing agent rFVIIa. The phase 2 trial results, which include the explorer5 trial in HA without inhibitors, support further development of concizumab as a prophylactic treatment for all hemophilia patients and have guided selection of the phase 3 dosing regimen.
Shapiro:Sangamo Biosciences Inc: Consultancy, Other: Clinical Research Protocol with the company; Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Clinical Research Protocol with the company; Novo Nordisk Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Research Protocol with the company; Bioverativ: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Research Protocol with the company; OPKO: Other: Clinical Research Protocol with the company; Octapharma: Other: Clinical Research Protocol with the company; Prometic Life Sciences: Consultancy; Shire/Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Clinical Research Protocol with the company, Research Funding; Bayer: Other: Clinical Research Protocol with the company; Kedrion Biopharma: Other: Clinical Research Protocol with the company; Agios: Other: Clinical Research Protocol with the company; Prometic Bio Therapeutics: Other: Clinical Research Protocol with the company; BioMarin: Other: Clinical Research Protocol with the company; Daiichi Sankyo: Other: Clinical Research Protocol with the company; Glover Blood Therapeutics: Other: Clinical Research Protocol with the company; Novartis: Other: Clinical Research Protocol with the company; Pfizer: Other: Clinical Research Protocol with the company; American Thrombosis and Hemostasis Network: Membership on an entity's Board of Directors or advisory committees. Castaman:Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kedrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; CSL Behring: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Uniqure: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Werfen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda (SHIRE): Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Cepo:Novo Nordisk A/S: Employment. Hvitfeldt Poulsen:Novo Nordisk: Other: Clinical trials - investigator, Funding meetings and congresses; Bayer Health Care: Other: Clinical trials - investigator, Funding meetings and congresses; Pfizer: Other: Funding meetings and congresses; Sobi: Other: Funding meetings and congresses. Hollensen:Novo Nordisk: Employment. Matsushita:Bioverative: Research Funding; Pfizer: Consultancy, Honoraria; KM biologists: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Honoraria; CSL: Consultancy, Honoraria; uniQure: Consultancy, Honoraria. Young:Bioverativ/Sanofi: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria; Freeline: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria; Kedrion: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria; Spark: Consultancy, Honoraria; Shire/Takeda: Consultancy, Honoraria; Uniqure: Consultancy, Honoraria. Zupancic-Salek:Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Biogen: Consultancy, Honoraria, Speakers Bureau; Sobi: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy, Honoraria, Speakers Bureau. Jimenez-Yuste:Bayer, CSL Behring, Grifols, Novo Nordisk, Octapharma, Pfizer, Roche, Sobi, Shire: Consultancy, Honoraria, Other: reimbursement for attending symposia/congresses , Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal