
Introduction
Emicizumab is a recombinant humanized monoclonal antibody that bridges factor IXa and Factor Xa and is administered via subcutaneous injection. After initial FDA approval for patients with hemophilia A (HA) and inhibitors, it was approved in October 2018 for prophylaxis in patients with HA without inhibitors of all ages. In clinical trials of subjects without inhibitors, emicizumab was studied only in those >12 years of age.
The primary objective of this study was to report our "real-world", post-licensure experience with emicizumab across a more heterogenous patient population, comparing annualized bleeding rates prior to and after initiating emicizumab. Secondary objectives included evaluating for serious adverse events including death, thrombosis or drug discontinuation.
Methods
We conducted a multi-center observational study at 3 federally funded Hemophilia Treatment Centers: Children's Hospital of Philadelphia, Virginia Commonwealth University and Children's National Health System. Inclusion criteria included patients with HA initiated on emicizumab prior to May 15th 2019. Data extraction included: demographics, diagnosis, prior HA history (inhibitor, prophylaxis treatment regimen), emicizumab dosing data, bleeding events (all bleeds, treated bleeds, joint bleeds, traumatic bleeds) and thrombotic events from the 6 months prior to emicizumab until July 15th 2019. Annualized bleeding rates (ABR) were calculated to account for variable follow up. Wilcoxon Sign-Rank Difference Test was used to test the difference in number of bleeds and McNemar's test was used to test the difference in proportions of patients with ABR=0.
Results
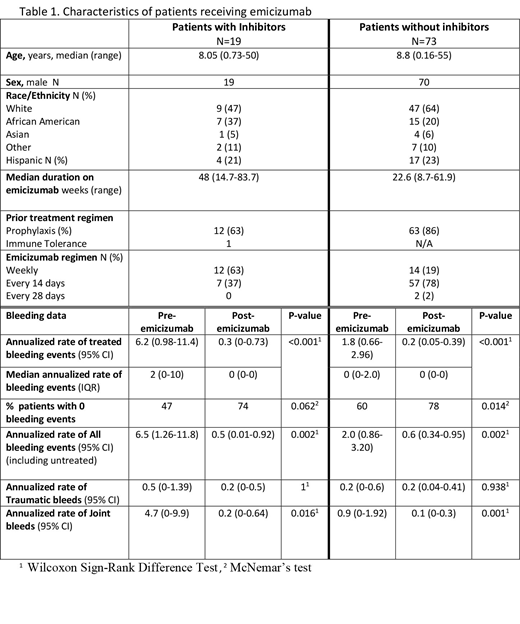
Ninety-two patients met inclusion criteria: 89 male and 89 with severe HA (3 moderate HA) (Table 1). Age at initiation of emicizumab ranged from 5 weeks to 55 years; median 8.6 yrs (IQR 4.8-13.5 yrs). Nineteen patients had an active inhibitor at the time of starting emicizumab; the remaining 73 did not have an inhibitor, and most (86%) were on prophylaxis prior to emicizumab. Sixty-two patients were < 12 years of age (13 with inhibitors). Median duration of emicizumab therapy was 48 weeks (inhibitors) and 22 weeks (non-inhibitors), with a total follow up of 53.3 patient years.
In patients with inhibitors, the ABR (treated bleeds) prior to emicizumab was 6.2 events (95% CI, 0.98 to 11.4) compared to 0.3 events (95% CI, 0 to 0.73) on emicizumab, p=0.002. In subjects without inhibitors, the ABR prior to emicizumab was 1.8 events (95% CI, 0.66 to 2.96) compared to 0.22 events (95% CI, 0.05 to 0.39) on emicizumab, p<0.001. Additional data on bleeding events is provided in Table 1. The proportion of patients with ABR=0 increased in both inhibitor (from 46% to 74%, p=0.06) and non-inhibitor subjects (from 60 to 78%, p=0.015).
All patients received 1.5 mg/kg weekly x 4 loading doses, followed by either weekly (27%), every other week (70%), or monthly (3%) dosing; patients with inhibitors were more likely to receive weekly dosing.
No patients who initiated emicizumab discontinued the drug or developed a neutralizing antibody to FVIII or emicizumab, although patients have not been consistently screened for these antibodies. No thrombotic or thrombotic microangiopathy events or death have occurred.
Conclusions
Our favorable clinical experience with emicizumab post-licensure in patients with HA is similar to that reported in the clinical trials. Although excluded from the clinical trials, patients with HA without inhibitors < 12 years accounted for the majority (67%) of our cohort. As anticipated, these younger patients appear to experience the same benefit as patients > 12. No serious drug-related adverse events were reported, although the overall study period is relatively short. Ongoing follow up of these patients, and others, will be important to assess the ongoing safety and efficacy profile of this novel therapy. Updated data on this cohort will be presented at the ASH meeting
Guelcher:NovoNordisk: Other: Advisory Board; Octapharma: Other: Advisory Board; Takeda: Other: Advisory Board; Genetech: Other: Advisory Board. Butler:pfizer: Other: Advisory board; Hema-Biologics: Consultancy; genetech: Other: Advisory board. Guerrera:Genetech: Other: Advisory Board; Kendrion: Other: Advisory Board; Shire: Other: Advisory Board; Bayer: Other: Advisory Board; Bioverativ: Consultancy; Pfizer: Other: Advisory Board; Novo Nordisk: Consultancy. Raffini:Roche: Other: Advisory Board; Bayer: Other: Advisory Board; CSL Behring: Other: Advisory Board.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal