Introduction:
Patients with severe factor VIII (FVIII) deficiency are defined as having a FVIII level of <1%. Approximately 30% of FVIII deficient patients will develop inhibitors which significantly worsen morbidity and increase mortality. The treatment of patients with inhibitors currently involves using activated prothrombin complex concentrate (aPCC) and recombinant activated FVII (rFVIIa), the so-called bypassing agents (BPA). However, there remains a significant unmet need for improved medications to prevent bleeding in inhibitor patients. Recently, the novel agent, emicizumab, has demonstrated a significant reduction in the rate of bleeding compared to BPA. What remains unclear, however, is the degree to which emicizumab corrects the clotting defect, and the objective of this study was to address this question.
Methods
Patients with mild/moderate hemophilia (group 1) and severe FVIII deficient patients with inhibitors and on steady-state emicizumab (group 2) participated in the study. Blood was drawn from both groups in the non-bleeding state and with no recent factor infusion and assayed for FVIII activity (group 1) and thrombin generation (both groups). Factor VIII activity was measured with a one-stage clotting assay while TGA was performed using the calibrated automated thrombogram on platelet poor plasma using the PPP low reagent. First, linear regression was utilized to model the FVIII levels as a function of the endogenous thrombin potential (ETP) and peak thrombin values for patients in group 1. Then, we used the ETP and peak thrombin results of the emicizumab patients (group 2) with the calibration curve to calculate their predicted FVIII level. Association between patient weight and their predicted FVIII levels were examined and evaluated by linear regression.
Results
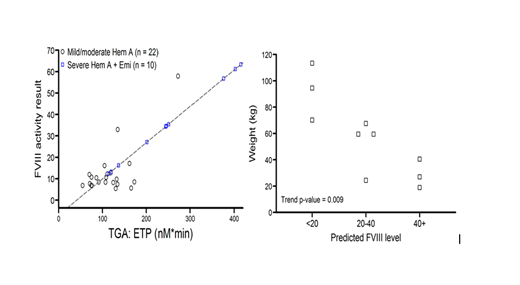
Twenty three patients with moderate and mild FVIII deficiency (levels 1-40%) and 11 patients with severe FVIII deficiency and inhibitors on emicizumab were enrolled. We had insufficient data for one patient in each group and therefore data is presented for 22 and 10 patients for group 1 and 2, respectively. Figure 1 (below on the left) demonstrates the raw data and the regression line for the FVIII levels versus ETP for group 1 and the squares represent the predicted FVIII levels for patients on emicizumab based on their actual ETP. Peak thrombin results are not shown in the figure but are similar to the ETP data. Figure 2 (below on the right) shows the relationship between the inhibitor patient's weight and their predicted FVIII level demonstrating that the heavier patients had lower predicted FVIII levels.
Discussion
Determining the degree of correction of the clotting defect for patients on emicizumab is important in order to make good clinical decisions regarding activities, response to therapy, and perhaps even surgery. As of yet, no studies have been performed to address this issue. In this study, we made a calibration curve of native FVIII levels and ETP and peak thrombin in mild/moderate hemophilia A patients, and then used the ETP and peak thrombin results of inhibitor patients on emicizumab to predict their FVIII level. All the patients on emicizumab had predicted FVIII levels above 10% with most having levels above 20%. The wide variability in the predicted FVIII level was tightly correlated to weight with the heaviest patients having the lowest FVIII predicted levels. It is unclear at this time why this may be the case, and further data will be collected to assess this relationship. Few bleeding events were reported in our inhibitor patients such that in this small group we cannot correlate the results to bleeding. Moving forward, understanding the correction of the clotting defect of non-replacement therapies is an important goal.
Young:Uniqure: Consultancy, Honoraria; Freeline: Consultancy, Honoraria; Grifols: Consultancy, Honoraria; Kedrion: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria; Spark: Consultancy, Honoraria; Shire/Takeda: Consultancy, Honoraria; Genentech/Roche: Consultancy, Honoraria, Research Funding; Bioverativ/Sanofi: Consultancy, Honoraria; CSL Behring: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal