Background:
BAY 94‐9027 is a B-domain deleted recombinant factor VIII (FVIII) that is site-specifically PEGylated with a 60 kDa (2×30 kDa) polyethylene glycol to extend its half-life. Efficacy and safety of BAY 94-9027 as prophylactic (PPX) and on-demand (OD) therapy for patients with severe hemophilia A (HemA) were demonstrated in the phase II/III PROTECT VIII trial (NCT01580293) and its Extension. BAY 94-9027 has been approved in the US, EU, Japan and Canada for previously treated patients ≥12 years old. Extended dosing (e.g. once-weekly PPX) is an attractive therapeutic approach for patients previously treated OD. This post hoc analysis was conducted to confirm the anticipated bleeding and quality of life (QoL) outcomes among patients who were previously treated with OD FVIII and switched to BAY 94-9027 PPX in PROTECT VIII.
Patients/Methods:
PROTECT VIII was a partially randomized, open-label trial of 134 males aged 12-65 years with HemA (FVIII <1%) and ≥150 FVIII exposure days. At enrolment, prior OD patients could select PPX or continue OD therapy. PPX patients received BAY 94-9027 25 IU/kg twice weekly (2×W) for a 10-week run-in period. Patients with ≤1 spontaneous joint or muscle bleeds during this period were randomized to 45‒60 IU/kg every 5 days (E5D) or 60 IU/kg every 7 days (E7D) for the main 26-week study period. Patients enrolling after the randomization arms were full, or with ≥2 bleeds in the run-in period, received 30-40 IU/kg 2×W. Patients completing the main study could enter an extension and continue, or switch regimens. Patients switching after Extension start were evaluated as a 'variable' group (VAR). The primary efficacy outcome was annualized bleeding rate (ABR). QoL was assessed using the hemophilia-specific health-related quality of life questionnaire for adults (Hemo-QoL-A) and the Work Productivity and Activity Impairment (WPAI) questionnaire. All analyses were descriptive and reported outcomes by pre-study treatment regimen.
Results:
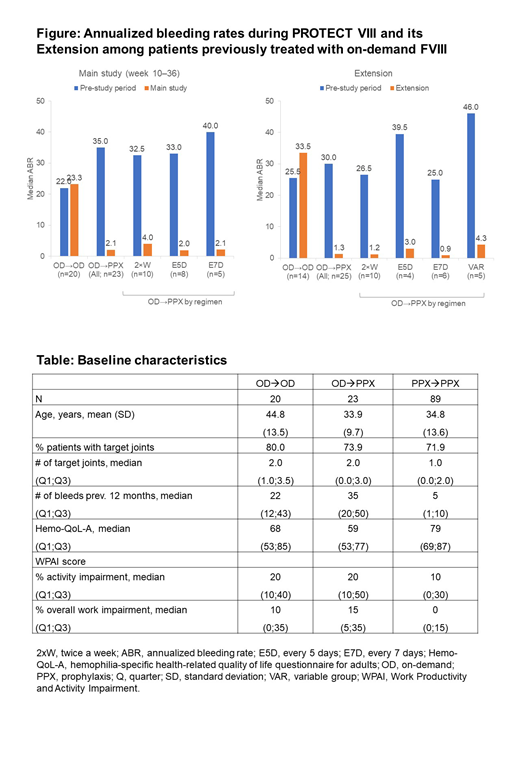
Of 43 patients on prior OD therapy, 20 selected OD BAY 94-9027 [OD→OD] and 23 selected PPX BAY 94-9027 [OD→PPX] in the main PROTECT study. Of 39 patients on prior OD therapy who continued into the Extension, 14 continued OD, and 25 switched to PPX [OD→PPX], including 3 patients who switched from OD at the Extension start. A total of 89 patients on prior PPX therapy received PPX during the main study [PPX→PPX] and 82 continued to receive PPX during the Extension. At data cut-off (Feb 2017), median time in the study was 3.9 years (3.2 years in the Extension). See Table for baseline characteristics.
At the end of the main study, median ABR was 23.4, 2.1, and 2.1 in the OD→OD, OD→PPX and PPX→PPX groups, respectively. During the Extension, median ABR was 33.5, 1.3 and 1.6 in the OD→OD, OD→PPX and PPX→PPX groups, respectively. Robust improvements in median ABR were observed for patients previously on OD treatment in both the main study and Extension, irrespective of which PPX regimen they received (Figure).
Hemo-QoL-A total score was maintained or improved from baseline during the main study all PPX regimens with greater benefit among OD→PPX (median change from baseline: 2.5 for all PPX and 4.3 for OD→PPX), and greatest improvement in OD→E7D PPX (median change from baseline: 13.8; almost twice the minimal clinically important difference [7-8]). OD patients prior to study entry also saw higher benefit in WPAI activity and work impairment sub-scores compared with prior PPX patients (mean changes from baseline: -15.9 vs -4.9 and -16.4 vs -1.7, respectively). As of August 2018, E7D PPX had a similar number of infusions per year to OD treatment (52 vs 47) and double median consumption (3048 vs 1394 IU/kg/year for E7D and OD, respectively).
Conclusions:
Despite entering PROTECT VIII with higher ABR than PPX→PPX patients, OD→PPX patients experienced a major decrease in ABR with BAY 94-9027 treatment during the first 6 months, achieving a similar rate to that observed in patients who received PPX before the trial. OD→PPX patients experienced larger improvements in QoL and WPAI sub-scores from baseline compared with PPX→PPX patients. Prior OD patients who switched to BAY 94‐9027 E7D had the greatest improvement in QoL. Further, the number of infusions/year was only slightly higher with E7D dosing than with OD treatment, and the difference in median consumption between PPX and OD was smaller than seen in previous studies.
Ducore:BioMarin: Research Funding; HEMA Biologics: Consultancy, Honoraria; Shire: Consultancy, Honoraria; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioverativ: Research Funding; Spark Therapeutics: Research Funding; Bayer: Consultancy, Honoraria, Other: speaker (not bureau). Lalezari:Bayer: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Teva: Consultancy, Honoraria; Bayer: Speakers Bureau; Pfizer: Speakers Bureau. Santagostino:CSL Behring: Speakers Bureau; Bayer: Speakers Bureau; Grifols: Speakers Bureau; Bioverativ Sanofi: Speakers Bureau; Kedrion: Speakers Bureau; Novo Nordisk: Speakers Bureau; Octapharma: Speakers Bureau; Pfizer: Speakers Bureau; Shire/Takeda: Speakers Bureau; Sobi: Speakers Bureau; UniQure: Speakers Bureau; Roche: Speakers Bureau; Spark: Speakers Bureau. Ahuja:XaTexk Inc.: Consultancy, Patents & Royalties, Research Funding; Rainbow Children's Foundation: Research Funding; Genentech: Consultancy; Biovertiv Sanofi: Consultancy; Bayer: Consultancy. Maas Enriquez:Bayer AG: Employment. Tueckmantel:Bayer: Employment. Reding:Takeda: Consultancy, Honoraria, Speakers Bureau; Biomarin: Research Funding; Sanofi Genzyme: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal