Introduction: Emicizumab is a novel humanized bispecific antibody that mimics the function of activated coagulation factor VIII (fVIII). It has significantly changed the management of patients with hemophilia A and inhibitors by achieving baseline hemostatic control. Based on the HAVEN studies, emicizumab markedly reduces annualized bleeding rates and is FDA-approved for prophylaxis in hemophilia A patients of all ages, regardless of inhibitor status. In the HAVEN2 interim analysis, only 3/57 pediatric patients receiving emicizumab prophylaxis required treatment for an acute bleeding event after a 9-week median observation time. We report 3 patients with severe hemophilia A and a history of inhibitors receiving emicizumab prophylaxis with severe or refractory bleeding episodes to highlight the importance of vigilance and surveillance of children with severe hemophilia A on emicizumab.
Methods: This retrospective analysis includes patients between 0-21 years of age with severe hemophilia A (fVIII activity < 1%) receiving emicizumab prophylaxis and admitted for the management of an acute bleeding episode following emicizumab's FDA approval in November 2017. Patients were followed at the Pediatric Hemophilia Treatment Center at the Hemophilia of Georgia Center for Bleeding & Clotting Disorders of Emory and the St. Jude Affiliate Clinic at Novant Health Hemby Children's Hospital. Data collected included demographics, past medical history including inhibitor status, bleeding history, and treatment modalities, and details regarding the presentation, management, and outcome of acute severe bleeding events. Due to the nature of the study, descriptive statistics were primarily used for data analysis.
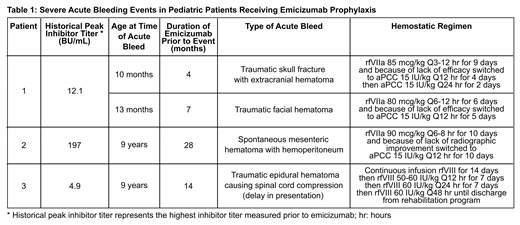
Results: Three patients with severe hemophilia A receiving emicizumab prophylaxis were admitted for the management of 4 severe bleeding episodes. All patients had a history of a fVIII inhibitor. Three of the 4 bleeding episodes were trauma-induced while 1 occurred spontaneously. For the traumatic episodes, all patients presented with worsening symptoms approximately 1 week following the inciting event. All patients had a normal aPTT at the time of presentation, ruling out a significant anti-drug antibody (emicizumab level not available). A patient with a low-titer inhibitor developed an epidural hematoma following a trampoline injury and was treated with continuous infusion of recombinant factor VIII (rfVIII), adjusting the rate to achieve chromogenic fVIII activity of 100% for 14 days. Following 14 days, he was started on rfVIII 50 IU/kg Q12 hours with a goal fVIII activity of 50%. His rfVIII dosing interval was gradually weaned to every other day while in inpatient rehabilitation. As outlined in Table 1, the remaining 3 bleeding events were initially managed with recombinant activated factor VII (rfVIIa) dosed at 80-90 mcg/kg/dose with escalating frequency for an average of 8 days. However, due to lack of improvement, treatment was changed to low-dose activated prothrombin complex concentrates (aPCC; 10-15 IU/kg/dose Q12-24 hours for an average of 7 days). In all 3 of these events, the hematomas improved after treatment with aPCC. No patient experienced thrombotic microangiopathy, thrombosis, or had evidence of DIC while receiving these treatment regimens.
Discussion/Conclusion: Pharmacokinetic analysis of emicizumab suggests that following the standard 4-week loading phase, trough plasma emicizumab concentrations obtained prior to a 1.5 mg/kg once weekly maintenance dose correlates with at least 10-15 IU/dL equivalent fVIII activity. This degree of thrombin generation should be sufficient to prevent severe spontaneous bleeding episodes in most patients. However it does not preclude significant trauma-induced bleeding or spontaneous bleeding in inhibitor patients. Based on our cases, providers should maintain a high index of suspicion for acute bleeding in patients receiving emicizumab prophylaxis. Serious bleeding events, although rare, may have a more insidious onset in patients receiving emicizumab. Furthermore, despite the baseline hemostasis achieved with emicizumab, acute bleeding events may still require aggressive therapy. Our cases suggest that low-dose aPCC or continuous infusion fVIII may be feasible options for treating acute bleeding events in patients with hemophilia A and inhibitors receiving emicizumab prophylaxis.
Zimowski:Pfizer: Research Funding; National Hemophilia Foundation: Other: Medical Loan Reimbursement, Research Funding. Batsuli:Octapharma: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Genetech: Membership on an entity's Board of Directors or advisory committees. Bryant:Novo Nordisk: Other: PI on Novo Nordisk sponsored Studies. McDaniel:Genentech: Membership on an entity's Board of Directors or advisory committees. Tickle:National Hemophilia Foundation: Research Funding. Meeks:Bayer: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Takeda-Shire: Membership on an entity's Board of Directors or advisory committees; HEMA Biologics: Membership on an entity's Board of Directors or advisory committees. Sidonio:Genetech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda-Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioverativ: Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Grifols: Membership on an entity's Board of Directors or advisory committees, Research Funding; Biomarin: Membership on an entity's Board of Directors or advisory committees; Uniqure: Membership on an entity's Board of Directors or advisory committees; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees; Kedrion: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal