Introduction: It has been shown that megakaryocytes (MKs) can develop from a subset of MK-biased hematopoietic stem cells (HSCs). We also demonstrated that an active form of tyrosyl tRNA synthetase (YRS) has ex-translational activities that stimulate rapid platelet production in thrombocytopenic mice. Remarkably, YRS stimulates the development of MKs expressing the stem cell marker, Sca-1, and the monocyte/macrophage marker, F4/80. Thus, we hypothesized that YRS treatment mimics stress-induced megakaryopoiesis and Sca-1 may be a marker for MKs/platelets derived from MK-biased HSCs under inflammatory stress conditions. Accordingly, YRS does not induce Sca-1+ MKs in type I interferon (IFN-I) receptor knockout mice, suggesting a role of IFNs in the induction of Sca1+ MKs.
Methods: We used a transgenic mouse strain expressing EGFP under the transcriptional regulatory elements of the Sca-1 gene (Sca-1-EGFP Tg) as a sensitive marker of Sca-1+ MKs. To compare transcriptional profiles, we sorted from mouse bone marrow (BM) EGFP+F4/80+CD41+mGPIbα+ and EGFP-F4/80-CD41+mGPIbα+ cells - representing Sca-1+ MKs and Sca-1- (conventional) MKs, respectively - and performed RNA-Seq analysis. To investigate the mechanism of Sca-1+ MK induction, mouse BM cells were treated with a synthetic TLR7 agonist and MKs were analyzed by flow cytometry. To elucidate the role of Sca-1+ MKs in thrombopoiesis, we infected Sca-1-EGFP Tg mice with lymphocytic choriomeningitis virus (LCMV) Armstrong strain, and analyzed the percentage of EGFP+ platelets and expression of IFN-stimulated genes.
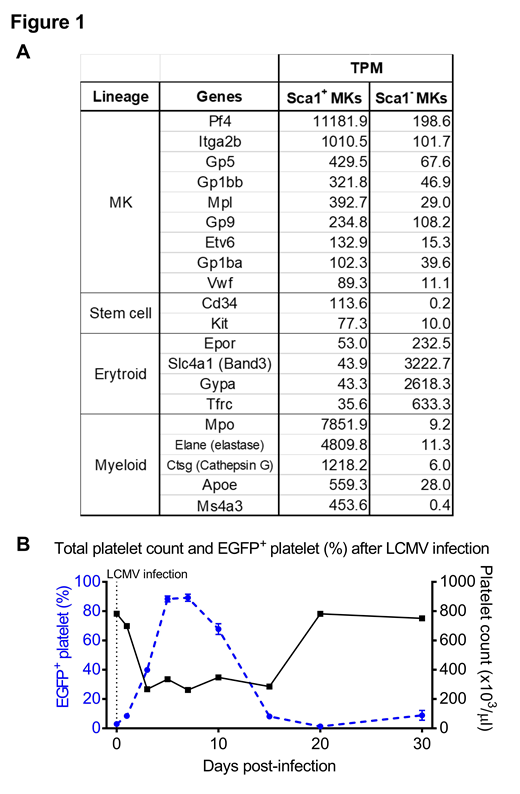
Results: We found that expression of MK-specific mRNAs is ~10-fold higher in Sca1+ than Sca1- MKs. As shown in Figure 1A, Sca1+ MKs highly expressed myeloid-related genes (Mpo, Elane, and Ctsg) and stem cell-related genes (Cd34 and Kit); in contrast, Sca1- MKs highly expressed erythroid lineage genes, consistent with origin from a common megakaryocyte-erythroid progenitor (MEP). Expression of IFN-stimulated genes, such as Ifitm1/2/3, is upregulated in Sca-1+ MKs, suggesting the involvement of IFN-I signaling in Sca-1+ MK induction. TLR7 typically recognizes single-stranded viral RNA and stimulates IFN-I production. When Sca-1-EGFP Tg BM cells were cultured in the presence of Gardiquimoid (1 µg/ml), a synthetic TLR7 agonist, Sca1- MKs were reduced and Sca1+ MKs were markedly increased compared to control vehicle treatment (9442 ± 1465 vs 4201 ± 1100). Notably, the proportion of high-ploidy cells was greater in Sca1+ thanSca1- MKs. Moreover, when Sca-1-EGFP Tg mice were infected with LCMV, which cause IFN-I-dependent thrombocytopenia, the percentage of EGFP+ platelets was markedly increased on day 3 (40.0 ± 3.7%) and peaked at day 7 (89.2 ± 5.4%) post-infection as compared to before (3.0 ± 1.0%) (Figure 1B), with 100-fold increase in the EGFP mean fluorescence intensity. Expression of Ifitm3 in platelets was also increased at day 10 post-infection. These results are consistent with our original hypothesis that YRS mimics inflammatory stress conditions and Sca1+ MKs are induced as a consequence of TLRs activation and IFN-I signaling.
Conclusions: Our findings indicate that TLR7 activation and IFN-I signaling shift MK generation from Sca-1- to Sca-1+ progenitors, which rapidly develop to high-ploidy Sca-1+ MKs that may accelerate recovery from thrombocytopenia. Accordingly, under inflammatory stress conditions, such as viral infection, the majority of circulating platelets originate was from Sca-1+ MKs. The high expression of Ifitm proteins, which are known to inhibit cellular entry by viruses, in Sca-1+ MKs/platelets may contribute to their role in host defense.
Aiolfi:MERU-VasImmune, Inc: Other: Stock option. Kanaji:MERU-VasImmune, Inc: Other: Stock option. Schimmel:aTyr Pharma: Consultancy, Equity Ownership, Patents & Royalties. Ruggeri:MERU-VasImmune Inc.: Equity Ownership, Other: CEO and Founder. Kanaji:MERU-VasImmune, Inc: Other: Stock option.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal