Background
Allogeneic hematopoietic stem cell transplantation (alloHSCT) has been established as a powerful treatment modality for patients with hematological malignancies. The graft-versus-leukemia effect, however, is strongly associated with the occurrence of graft-versus-host disease (GVHD) and subsequent transplant-related mortality (TRM). Several strategies are applied in order to prevent GVHD following T-cell replete alloHSCT including conventional immunosuppression (CIS) with post-transplant administration of cyclosporine A (CyA) and mycophenolic acid (MA), or post-transplant cyclophosphamide (PT-Cy) either or not combined with CIS. Studies in haplo- and HLA matched donor transplantation have shown that PT-Cy is well tolerated and associated with low rates of severe GVHD and TRM. However, evidence from randomized clinical trials on the efficacy of PT-Cy as compared to CIS in the setting of HLA matched alloHSCT is scarce.
Aims
In the present prospective randomized, multicenter, phase III trial we set out to compare a PT-Cy based immunosuppressive regimen with CIS and address the question whether PT-Cy would be associated with improved GVHD-free/relapse-free survival (GRFS). Endpoints included time to acute and chronic GVHD, progression free survival (PFS), GRFS, overall survival (OS), and adverse events.
Methods
Hematological patients (pts) with a matched related donor or at least an 8 out of 8 matched unrelated donor were included. Pts randomized for the CIS regimen received CyA twice daily until day +120 followed by tapering until day +180 and MA 16 mg/kg twice daily with a maximum dose of 2160 mg a day until day 84 post-transplant. Pts randomized for PT-Cy received 50 mg/kg of cyclophosphamide on day +3 and +4 combined with CyA from day +5 until day +70.
Results
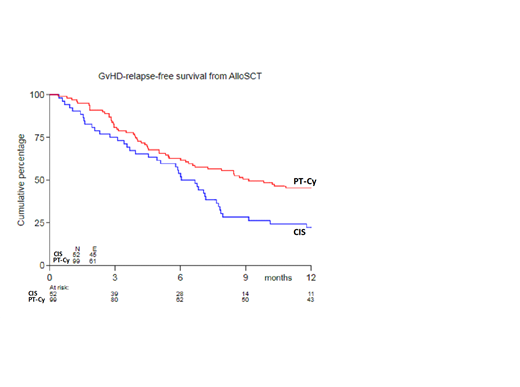
A total of 160 pts was randomized 1:2 between CIS and PT-Cy, of whom 94% proceeded to transplant (52 versus 99 pts). Median age was 58 years (range: 20-70), 66% were male. Two pts received myeloablative conditioning. The donor type was matched related in 31% and matched unrelated in 69% of pts. Transplants were derived from peripheral blood in 97% of pts and consisted of median 6.14x106/kg CD34+ cells/kg (range: 1.36-19.4) and median 230x106/kg CD3+ T cells (range: 0-519). Baseline patient and transplantation characteristics were equally distributed between the two treatment arms. The cumulative incidence (CI) at six months of grade II-IV acute GVHD was 48% in recipients of CIS versus 32% following PT-Cy (SHR 0.52, 95%CI 0.31-0.87, p=0.014), and grade III-IV 12% versus 6%. In recipients of PT-Cy, acute GVHD was generally limited to stage 1 skin involvement, whereas more severe skin involvement and bowel involvement were observed following CIS. The two-year CI of chronic extensive GVHD was 50% in recipients of CIS versus 19% following PT-Cy (SHR 0.38, 95%CI 0.21-0.67, p=0.001). The three-year estimate of PFS was 60% (44%-73%) and 58% (46%-67%). The three-year CI of progression/relapse was 26% in the CIS arm versus 32% in the PT-Cy arm. The three-year estimate of OS was 69% (53%-80%) and 63% (52%-73%). The one-year estimate (95% confidence interval) of GRFS was 22% (12%-34%) and 45% (35%-55%), respectively.
Conclusion
Use of high-dose PT-Cy results in a significant reduction in severe acute and chronic GVHD without affecting relapse, thereby resulting in improved GRFS. Hence, a more intensified immunosuppression regimen with PT-Cy might be preferred as GVHD prophylaxis in the setting of RIC alloHSCT.
Nur:Novartis Pharmaceuticals: Consultancy. Maertens:Cidara: Other: Personal fees and non-financial support; Gilead Sciences: Other: Grants, personal fees and non-financial support; Amplyx: Other: Personal fees and non-financial support; Merck: Other: Personal fees and non-financial support; Pfizer: Other: Grant and personal fees; Astellas Pharma: Other: Personal fees and non-financial support; F2G: Other: Personal fees and non-financial support. Deeren:Alexion, Amgen, Janssen, Roche, Sunesis, Takeda, Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal