Abstract
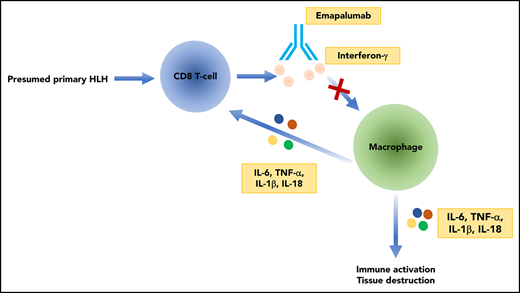
Emapalumab is a fully human immunoglobulin G1 monoclonal antibody directed against interferon-γ (IFN-γ), which in November 2018 received the first global approval for the treatment of pediatric and adult patients with primary hemophagocytic lymphohistiocytosis (HLH) with refractory, recurrent, or progressive disease or intolerance to HLH therapy. This review will highlight the pathophysiology of primary HLH, the therapeutic rationale for use of IFN-γ–targeting therapy, and potential limitations to its broader use in the treatment of HLH.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a hyperinflammatory state characterized by immune dysregulation. Certain genetic lesions are enriched in HLH patients, particularly familial HLH. Additionally, certain environmental triggers, including viral infection, can predispose to HLH. Primary HLH presents in young children, usually before 1 year of age, and tends to be a Mendelian disorder with defects in cytolytic function of cytotoxic T cells or natural killer cells, as well as genes involved in Epstein-Barr virus (EBV) clearance. Genes commonly mutated in primary HLH include PRF1, UNC13D, STX11, and STXBP2.1-3 Mutations in these genes constitute different subtypes of familial HLH.3 Other disorders, including Chediak-Higashi syndrome, which have mutations in the lysosomal transporter LYST, as well as Griscelli type 2 syndrome (mutated RAB27A) and various X-linked lymphoproliferative disorders also give rise to HLH.4 In contrast, secondary HLH, more commonly seen in adults, is due to an exaggerated immune response to infections such as EBV, cytomegalovirus (CMV), or immune dysregulation in the context of malignancy or rheumatologic disease. HLH that arises in the background of rheumatologic disease is referred to as macrophage activation syndrome–HLH.5,6
There are few good models for this disease and the heterogeneity of triggering etiologies also provides additional complexity that is not accounted for by existing models of HLH. Patients with HLH have elevations in numerous cytokines including interleukin-2 (IL-2), IL-6, IL-10, IL-18, as well as interferon-γ (IFN-γ), in the context of hyperactivation of T-lymphocytes and defective natural killer cell function.7
Treatment of adults and children with HLH centers around suppressing the life-threatening inflammation that characterizes the disorder. The HLH-94 protocol is commonly used as first-line treatment and involves weekly treatments with dexamethasone and etoposide, as well as intrathecal methotrexate and hydrocortisone for patients with evidence of central nervous system involvement. Despite treatment, mortality is observed in ∼50% of adults and children.8,9 Furthermore, treatment with topoisomerase inhibitors, such as etoposide, can have long-term sequelae including an increased risk of therapy-related leukemia. Hematopoietic stem cell transplantation (HSCT) is considered in individuals with a mutation in a gene implicated in HLH, as well as individuals with relapsed, refractory disease.9 Emapalumab represents the first approval of a cytokine-targeting therapy in the treatment of HLH.10
Mechanism of action
Emapalumab is a fully human immunoglobulin G1 monoclonal antibody that noncompetitively inhibits IFN-γ. It binds with high affinity (Kd = 1.4 pM) to both free IFN-γ as well as IFN-γ bound to its receptor.10
The relative importance of various cytokines and signaling pathways, including IFN-γ, has been investigated in animal models of HLH.11 One of the most commonly used models of HLH is the perforin null (Prf1−/−) mouse, which has impaired adaptive immunity due to deficient cytolysis. These mice develop an HLH-like pathology following stimulation with lymphocytic choriomeningitic virus.12,13 Administration of antibodies to deplete CD8+ T cells, as well as neutralization of IFN-γ, rescued the HLH-like pathology and improved survival in these mice. In contrast, neutralization of multiple other cytokines such as tumor necrosis factor α, IL-12, IL-18, and macrophage– and granulocyte-macrophage–colony-stimulating factor had no effect on survival in this model.13 A model of secondary HLH involves the administration of the Toll-like receptor 9 agonist, cytosine guanine dinucleotide, with or without IL-10 receptor blockade in wild-type C57BL/6 mice. In this secondary HLH model, Toll-like receptor 9 stimulation in an Ifng−/− background results in improved anemia, decreased splenomegaly, and reduced hepatic inflammation but no improvement in hyperferritinemia or leukopenia.14 A similar attenuation of the HLH phenotype was seen with IFN-γ neutralization. These preclinical models provide a mechanistic basis for therapeutic targeting of IFN-γ in the context of primary and secondary HLH.14
However, as multiple cytokines play a role in the hyperinflammatory cytokine storm of HLH, targeting of a single cytokine may not be sufficient to ameliorate the tissue inflammation and organ dysfunction that accompany the disorder.14,15 The JAK/STAT pathway lies downstream of several cytokines that are elevated in HLH and represents an attractive therapeutic target to simultaneously abrogate the signaling of multiple cytokine pathways. JAK/STAT inhibition with the JAK1/2 inhibitor ruxolitinib has also been shown to decrease organomegaly, anemia, thrombocytopenia, tissue damage, and cytokine levels in the Prf1−/− murine model of HLH.16 Additionally, ruxolitinib and an IFN-γ–neutralizing antibody were compared in 2 murine models of HLH. Ruxolitinib and the IFN-γ–neutralizing antibody resulted in decreased inflammation-associated anemia, but ruxolitinib treatment also resulted in decreased T-cell and neutrophil activation and tissue infiltration.17 These murine data are also supported by several case studies demonstrating improved clinical and laboratory parameters in patients with refractory HLH treated with ruxolitinib, highlighting a role for investigating multicytokine-targeting immune-modulatory therapies.18-21
Clinical trials
The safety and efficacy of emapalumab was assessed in a phase 2/3 trial (NCT01818492) in pediatric patients with presumed primary HLH.22 The trial included 34 patients with presumed primary HLH and active disease who were treatment naive or had already received conventional HLH therapy and failed or were intolerant to standard treatments. The median age of the study population was 1 year of age. Genetic mutations were present in 79% of patients and baseline characteristics and distribution of disease mutations were similar among those who failed conventional therapy prior to study entry. Patients with secondary HLH due to a rheumatologic or malignant etiology were excluded as were patients with latent tuberculosis or active Mycobacterium, Histoplasma, Leishmania, or bacterial infections including Shigella, Salmonella, and Campylobacter. All patients in the trial received 1 mg/kg emapalumab by IV infusion every 3 to 4 days along with dexamethasone at 5 to 10 mg/m2 per day. Dosing of emapalumab could be increased to 10 mg/kg based on laboratory evidence of response. Patients were treated for 8 weeks or extension until HSCT. In addition, 22 of the original 34 patients were enrolled in a 1-year extension trial after HSCT.10,22
A phase 2/3 study (NCT03985423) to evaluate the efficacy, safety, and pharmacokinetics of emapalumab in patients with adult HLH is poised to begin, and will include adult patients with both malignancy- and nonmalignancy-associated HLH.
Efficacy
The primary end point for efficacy was overall response rate (complete or partial response or HLH improvement) at week 8 or the end of treatment (whichever occurred first). Secondary outcome measures included overall survival up to 18 months and the number of patients proceeding to HSCT. Complete response was defined as normalization of all HLH abnormalities whereas partial response was defined as improvement of ≥3 HLH abnormalities at least 50% from baseline.23
Among emapalumab-treated patients, the median time to response was 8 days and the overall response rate was 64.7% (95% confidence interval, 46% to 80%; P = .0031), with 26% achieving a complete response, 30% achieving partial response, and 7.4% achieving improvement in their HLH symptoms. Twelve-month survival was 69%, and 64.7% of patients proceeded to HSCT. Post-HSCT survival was 90.9% in patients who entered an extension phase after the completion of the study. Efficacy of emapalumab was also demonstrated in the subset of 27 patients among the 34 who failed conventional therapy. In this subset, the overall response rate was 63% (95% confidence interval, 42% to 81%; P = .0134) and 12-month survival was 73%.23
Adverse events
The 34 patients in this phase 2/3 trial received a median cumulative emapalumab dose of 25 mg/kg for a median of 59 days. Frequently encountered adverse reactions included infections (56%), hypertension (41%), infusion-related reactions (27%), and pyrexia (24%). Other less commonly seen adverse reactions, observed in 10% to 20% of patients, included hypokalemia, constipation, rash, abdominal pain, CMV infection, diarrhea, lymphocytosis, cough, irritability, tachycardia, and tachypnea. Treatment discontinuation occurred in 1 patient with disseminated histoplasmosis. Serious adverse reactions occurred in 53% of emapalumab recipients and included infections, gastrointestinal hemorrhage, and multiple organ dysfunction.23
Clinical use
Emapalumab is now approved for use in patients with adult or pediatric primary HLH that is refractory, recurrent, or progressive or in those who have intolerance to conventional therapy. Of note, the initial trial included no adult patients. Emapalumab is initiated at a dose of 1 mg/kg and is given as an IV infusion over 1 hour every 3 to 4 days. Dosing can be increased up to 10 mg/kg based on clinical response as assessed with changes in clinical and laboratory markers of HLH. Patients should also concomitantly receive dexamethasone (5-10 mg/m2 per day) for the duration of therapy.24
Patients should be evaluated for latent tuberculosis infection with an IFN-γ release assay or purified protein derivative placement prior to initiation of emapalumab. Patients are also recommended to receive prophylaxis for herpes zoster and Pneumocystis jirovecii for the duration of therapy with emapalumab. Monitoring for EBV, CMV, and adenovirus infections should occur every 2 weeks and repeat assessment for tuberculosis infection as clinically indicated. Additionally, live and attenuated vaccines should not be administered to patients on emapalumab for the duration of therapy and for a month following the last dose of treatment. There are no data to guide use of emapalumab in pregnant or lactating women, however, other published data suggest that limited amounts of therapeutic antibodies can be detected in breast milk.24
Current treatment of primary HLH involves the use of HLH-specific therapy based on the HLH- 94 protocol. This protocol involves the use of 8 weeks of induction therapy with etoposide and dexamethasone, along with intrathecal therapy for those with central nervous system symptoms. Patients who achieve response to HLH-specific therapy are considered for HSCT to achieve long-term cure. Long-term evaluation of the HLH-94 treatment protocol demonstrated a 54% 5-year survival for patients who received HLH-94 therapy and underwent HSCT.9 The data from the phase 2/3 trials resulting in the approval of emapalumab are limited by the small study size, as well as the narrow population studied (median age of 1 year old with primary HLH), and the relatively short-term of follow-up. However, it is encouraging that a large majority of patients who received emapalumab, either as initial therapy or second-line treatment, had a response and that the vast majority of patients were able to proceed to HSCT.
Conclusions
Emapalumab is the first cytokine-targeting therapy approved specifically for the treatment of HLH. This treatment involves the infusion of a monoclonal antibody targeting IFN-γ along with dexamethasone, and resulted in a high overall response rate in a small pediatric population of patients with primary HLH. This therapy represents a shift away from cytotoxic chemotherapy, toward more targeted immune modulation. However, the approval of emapalumab and our understanding of its efficacy and adverse events are derived from a small population of a narrow subset of pediatric patients with primary HLH. Furthermore, the data from the original trial have not been peer-reviewed or published outside of the US Food and Drug Administration (FDA) report.
It remains to be seen how efficacious this treatment will be in adult patients with HLH, for whom it was also approved, despite the lack of clinical trial data. Primary HLH is a disease of children, and the vast majority (if not all) of HLH in adults is secondary HLH. It remains to be demonstrated whether emapalumab can successfully treat patients with secondary HLH, although studies in the murine model of the disease suggest that it may. Furthermore, because nearly all pediatric patients treated with the drug were moved expeditiously to transplant, further studies will be needed to verify its efficacy as primary therapy for adult HLH. Consequently, until such studies are performed, emapalumab should not replace standard therapy for adult HLH. Additionally, because robust randomized clinical trial data are lacking, emapalumab should not replace standard first-line therapy in the treatment of HLH.
Emapalumab opens up the strategy of cytokine targeting in HLH, but investigation in murine models suggests that inhibiting multiple cytokine-signaling pathways simultaneously, such as with JAK1/2 inhibition, may result in enhanced efficacy of treatment of HLH as compared with targeting IFN-γ alone. Building on the initial preclinical success of emapalumab, additional studies and additional cytokine-targeting therapies will certainly inform our understanding of the role of cytokine and immune modulation in HLH treatment and the optimal strategies that should be applied depending on the environmental and genetic context from which the disease arises.
Authorship
Contribution: M.V. and N.B. wrote and edited the manuscript.
Conflict-of-interest disclosure: N.B. is a co-investigator on a phase 2/3 clinical trial of emapalumab in adult patients with HLH (NCT03985423). M.V. declares no competing financial interests.
Correspondence: Nancy Berliner, Division of Hematology, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis St, Boston, MA 02115; e-mail: nberliner@bwh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal