In this issue of Blood, Sébert et al made the intriguing observation that cases with germline DEAD-box helicase 41 (DDX41) mutations represent a unique entity among adult myeloid neoplasms (MNs), often with distinct clinical and molecular features.1
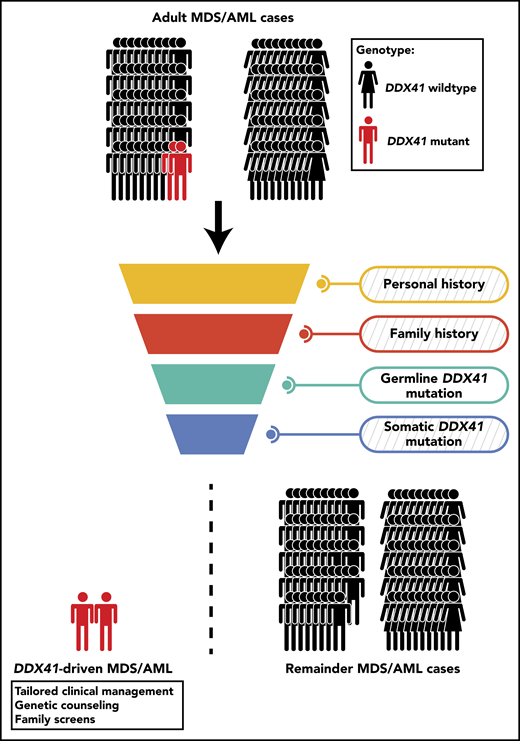
An unselected cohort of adult MDS/AML patients is screened to discover mutant DDX41-driven MDS/AML. Personal and family history of hematological disorders is recorded and reviewed for evidence of inherited cause, yet in the context of germline DDX41 mutations, this approach might prove inconclusive (indicated by the diagonally-striped background). Diagnostic assessment for germline and somatic DDX41 mutations could provide further indications, primarily when the germline DDX41 mutation is known to be causal or is supplemented by a somatic DDX41 mutation. Based on the results from Sébert et al, this approach would diagnose 2.4% of adult MDS/AML cases as being driven by causal germline DDX41 mutations.
An unselected cohort of adult MDS/AML patients is screened to discover mutant DDX41-driven MDS/AML. Personal and family history of hematological disorders is recorded and reviewed for evidence of inherited cause, yet in the context of germline DDX41 mutations, this approach might prove inconclusive (indicated by the diagonally-striped background). Diagnostic assessment for germline and somatic DDX41 mutations could provide further indications, primarily when the germline DDX41 mutation is known to be causal or is supplemented by a somatic DDX41 mutation. Based on the results from Sébert et al, this approach would diagnose 2.4% of adult MDS/AML cases as being driven by causal germline DDX41 mutations.
The advent of next-generation sequencing has profoundly improved our ability to define the genetic landscape of inherited hematological malignancies (HMs). Studies centered on familial HMs in combination with case studies of early-onset disease have been instrumental in identifying predisposing genetic variants. The list of genes with recognized predisposing variants is still growing.2,3 To accommodate this disease entity, the World Health Organization 2016 classification defined MNs with germline predisposition as a new subgroup.4 This distinction includes bone marrow failure syndromes (eg, Fanconi anemia), MNs with preceding cytopenias and platelet disorders (eg, inherited variants in GATA2, RUNX1, and ETV6) and a group of MNs lacking other preexisting dysfunctions (eg, inherited variants in CEBPA, and more recently, DDX41).
In this paper, the authors expand on the previous reports of germline DDX41 mutations5-8 by recognizing that cases carrying these germline mutations define a unique cluster within adult myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Germline DDX41 mutations predispose to late-onset MDS/AML, often at an age where these malignancies commonly manifest sporadically and frequently without preexisting hematological disorders. These features render diagnosing this inherited disease challenging compared with other leukemia predisposition syndromes, which are often associated with early-onset disease and defining clinical features. In this context, careful review of personal or family history for hematological disorders or other clinical stigmata often yields insufficient support to suspect inherited cause.6 Diagnostic assessment of germline DDX41 mutations is a better approach, yet requires the capacity to make distinctions between causal mutations from those without predisposing effect, which is currently hindered by the incomplete picture of the causal germline DDX41 mutation landscape.
Sébert et al screened a large cohort (n = 1385) of unselected adult MNs to determine the prevalence and characteristics of germline DDX41 mutations. Strict germline variant filtering yielded 21 causal variants detected in 33 patients, representing 2.4% of the total cohort. This frequency is remarkable, given the overall prevalence of inherited HMs within adult MNs, previously estimated at 5%, thereby rendering germline DDX41 mutations the largest contributor to inherited myeloid disease. Fifteen cases (46%) experienced mild cytopenias in the years before diagnosis, contrasting with Lewinsohn and colleagues who reported that antecedent cytopenias were rare in mutant DDX41-driven HMs.6 Only a limited number of cases had a family history of hematological disorders (n = 9, 27%). The previously unreported germline DDX41 variant p.G173R was the most prevalent, outnumbering germline variants previously reported as prevalent.5-8 This may indicate that the germline DDX41 variant spectrum can differ considerably among patient cohorts, potentially reflecting population differences. This is supported by the finding that the p.M1I and p.D140Gfs DDX41 germline variants are enriched in the Caucasian population, whereas the p.A500Cfs variant is exclusive to families of Asian descent.8 One possibility is that these variants represent geographically or population-restricted founder mutations, causing a greater heterogeneity of germline DDX41 mutations than previously appreciated.
Germline DDX41 mutations strongly predispose to somatic DDX41 lesions in the originally unaffected allele (79% of cases). Although concomitant germline and somatic DDX41 mutations appear to be common, their functional impact on the disease biology remains unclear, warranting further investigation. Additional recurrent somatic mutations were detected in ASXL1, EZH2, SRSF2, CUX1, and SETBP1, which have previously been more strongly associated to secondary AML rather than de novo AML.9 Quesada and colleagues7 noted that 60% of mutant DDX41-driven AML arose from antecedent MDS. Multilineage dysplasia, enrichment of mutations associated with secondary AML and personal history of cytopenias are suggestive that a proportion of mutant DDX41-driven AML cases reported by Sébert et al also arose from antecedent MDS. The overall mutational repertoire deviated from observations made by Polprasert et al5 and Quesada et al,7 who respectively showed that splice factor mutations are mutually exclusive to DDX41 mutations because of the role of DDX41 in precursor messenger RNA splicing, and that mutant DDX41-driven MNs are enriched for TP53 mutations. In contrast, Sébert et al reported splice factor mutations present in 6 cases (18%), while TP53 mutations were only present in 2 cases (6%). These discrepancies likely represent differences in patient population accrual as, for instance, Quesada et al7 noted that their cohort is enriched for high-risk MDS/AML.
At a first glance, based on the reported survival curves by Sébert et al, it seems that mutant DDX41-driven MNs have a relative favorable outcome compared to other cases of MN, yet the difference in survival is not significant (P = .97). This may be more reflective of the small number of included cases rather than the disease biology. In contrast, Polprasert et al5 reported a significant association between DDX41 lesions and poor survival. This divergence could be explained by the inclusion of MDS with 5q abnormalities spanning the DDX41 locus by Polprasert et al.5
Collectively, the finding that germline DDX41 mutations are common in a small fraction of MDS/AML warrants the integration of mutational analysis of this gene into routine diagnostics to inform long-term clinical management. Additionally, in the context of allogeneic stem cell transplantation, testing of related donors for the presence of these mutations is paramount to prevent donor-derived leukemia.10 A few fundamental questions remain unanswered. Previous work postulated that germline DDX41 mutations could predispose to lymphoid malignancies or, potentially, solid tumors.6 Although a few lymphoid malignancies were uncovered in this study, larger studies are needed to gain sufficient support that germline DDX41 mutations also predispose to lymphoid malignancies. On the other hand, questions concerning (1) the overall natural history of mutant DDX41-driven myeloid disease and (2) whether a complementing somatic DDX41 mutation, which sometimes present at low variant allele frequencies, is required for developing myeloid disease still need to be addressed. Studies reporting on germline DDX41 mutations have points of contention, such as, the presence or absence of cytopenia before disease or the enrichment or lack of certain classes of somatic mutations. Yet, all point out that reviewing the personal and family history for hematological disorders in combination with the screening for germline and somatic DDX41 mutations is a viable approach to distinguish mutant DDX41-driven disease from all other cases of adult MDS/AML (see figure). Longitudinal studies with large patient cohorts are required to better define the landscape of causal germline DDX41 mutations, establish the natural history of mutant DDX41-driven disease, and resolve the current points of contention, with the ultimate goal to improve diagnosis of this inherited hematological disease and further refine its clinical management.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal