Key Points
Concurrent DLBCL and FL has a similar outcome to that of GCB DLBCL; concurrent other indolent lymphoma does not worsen the DLBCL outcome.
Inclusion of patients with concurrent DLBCL and indolent lymphoma should be considered in clinical trials of newly diagnosed DLBCL.
Abstract
Some patients with diffuse large B-cell lymphoma (DLBCL) present with a concurrent indolent lymphoma at diagnosis. Their outcomes in the rituximab era are not fully defined. Using a prospectively followed cohort of 1324 newly diagnosed DLBCL patients treated with immunochemotherapy, we defined the prevalence, characteristics, and outcome of DLBCL with concurrent indolent lymphoma. Compared with patients with DLBCL alone (n = 1153; 87.1%), patients with concurrent DLBCL and follicular lymphoma (FL) (n = 109; 8.2%) had fewer elevations in lactate dehydrogenase, lower International Prognostic Index (IPI), and predominantly germinal center B-cell–like (GCB) subtype, whereas patients with concurrent DLBCL and other indolent lymphomas (n = 62; 4.7%) had more stage III-IV disease and a trend toward higher IPI and non-GCB subtype. After adjusting for IPI, patients with concurrent DLBCL and FL had similar event-free survival (EFS) (hazard ratio [HR] = 0.95) and a trend of better overall survival (OS) (HR = 0.75) compared with patients with DLBCL alone, but nearly identical EFS (HR = 1.00) and OS (HR = 0.84) compared with patients with GCB DLBCL alone. Patients with concurrent DLBCL and other indolent lymphomas had similar EFS (HR = 1.19) and OS (HR = 1.09) compared with patients with DLBCL alone. In conclusion, DLBCL patients with concurrent FL predominantly had the GCB subtype with outcomes similar to that of GCB DLBCL patients. DLBCL patients with concurrent other indolent lymphoma had similar outcomes compared with patients with DLBCL alone. These patients should not be summarily excluded from DLBCL clinical trials.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1361.
Disclosures
Associate Editor Laurie H. Sehn served as an advisor or consultant for AbbVie, Amgen, Celgene, Janssen Pharmaceuticals, Karyopharm Therapeutics, Merck, Roche/Genentech, Seattle Genetics, and TG Therapeutics and received grants for clinical research from Roche/Genentech. Author Brian K. Link served as an advisor or consultant for Genentech, Karyopharm, AbbVie, Gilead Sciences, and Celgene and received grants for clinical research from Genentech, Pharmacyclics, and Janssen Pharmaceuticals. Thomas E. Witzig served as an advisor or consultant for Incyte, Seattle Genetics, AbbVie, Morphosys, Spectrum, and Immune Design. Matthew J. Maurer served as an advisor or consultant for MorphoSys and received grants for clinical research from Celgene, NanoString Technologies, and Kite Pharma. Andrew L. Feldman served as an advisor or consultant for Infinity. Stephen M. Ansell received honoraria from WebMD and Research to Practice and received grants for clinical research from Bristol-Myers Squibb, Seattle Genetics, Affirmed Therapeutics, Regeneron, Pfizer, LAM Therapeutics, and Trillium Therapeutics. James R. Cerhan served as an advisor or consultant for Janssen Pharmaceuticals and received grants for clinical research from Celgene and NanoString Technologies. Grzegorz S. Nowakowski served as an advisor or consultant for Celgene, MorphoSys, and Genentech and received grants for clinical research from Celgene, NanoString Technologies, and MorphoSys. CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC and the remaining authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the prevalence and pathology pattern of concurrent indolent non-Hodgkin lymphoma (NHL) in patients with diffuse large B-cell lymphoma (DLBCL), according to a prospective cohort study
Identify the clinical characteristics of patients with DLBCL with or without concurrent indolent NHL, according to a prospective cohort study
Determine clinical outcomes of patients with DLBCL with or without concurrent indolent NHL, according to a prospective cohort study
Release date: October 17, 2019; Expiration date: October 17, 2020
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL). Approximately 10% to 15% of patients with newly diagnosed DLBCL also have concurrent indolent lymphoma at the time of diagnosis, for example, follicular lymphoma (FL) associated with DLBCL in the same tissue biopsy, or an indolent NHL in the bone marrow. FL and other indolent lymphomas can undergo histological transformation into a more aggressive lymphoma such as DLBCL. Therefore, some clinicians consider concurrent DLBCL and indolent lymphoma as early transformation of a previously undiagnosed indolent lymphoma.1,2 In contrast, other clinicians consider these cases as composite lymphoma, if the DLBCL and indolent NHL components are in the same nodal or extranodal tissue, or discordant lymphoma, if they are found in different tissue biopsies.3 Regardless, patients are managed for the most aggressive histology (DLBCL), with rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP) or R-CHOP–like immunochemotherapy. However, because these patients are generally excluded from phase 3 clinical trials, and when included are not reported separately, it is unclear whether these cases have a similar outcome to that of DLBCL following frontline immunochemotherapy.
A retrospective French study in the prerituximab era reported the characteristics and outcome of 60 DLBCL patients with an associated low-grade lymphoma at diagnosis, and these patients had inferior progression-free survival (PFS) but similar overall survival (OS) compared with a control group of DLBCL patients.1 A few other studies reported that low grade B-cell lymphoma involvement in the bone marrow did not negatively impact the outcome of DLBCL.4-6 In the rituximab era, several small retrospective series of concurrent DLBCL and FL (<60 cases each) have been described, with contradicting results for PFS and OS in comparison with DLBCL.7-10 Likewise, DLBCL with discordant bone marrow involvement of an indolent NHL has been retrospectively studied by several groups,11-14 most of which included <60 cases except for a recent MD Anderson study (n = 90),14 again with inconsistent results regarding PFS compared with DLBCL without bone marrow involvement.
In this study, we systematically characterized the prevalence, clinical and pathological features, and clinical outcomes of DLBCL with concurrent indolent NHL from a large prospective cohort of DLBCL patients treated in the rituximab era. We also compared these cases with those with presumably DLBCL alone, providing insights into their differences in clinical and pathological characteristics and outcomes, as well as important implications for clinical management and clinical trial inclusion/exclusion.
Methods
Patients
This study was reviewed and approved by the institutional review boards at Mayo Clinic and University of Iowa. All patients were from the Molecular Epidemiology Resource (MER) of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE). Full details of the MER, a prospective cohort study of lymphoma outcomes, have been published previously.15 Briefly, from 2002, consecutive patients with newly diagnosed lymphoma (within 9 months of first diagnosis) were offered enrollment into the MER. Consented and enrolled patients were treated per treating physician’s choice, and were followed systematically every 6 months for the first 3 years, and then annually thereafter. Pathology at initial diagnosis was reviewed and confirmed by a Mayo Clinic or University of Iowa hematopathologist and classified according to the World Health Organization (WHO) classification.16 Baseline clinical and pathological data were abstracted using a standard protocol. All treatment information, disease progression or relapse, and death were verified through medical record review. For this study, the MER database was queried in February 2018, and we included patients with DLBCL diagnosed between March 2002 and June 2015 who received frontline R-CHOP or R-CHOP–like immunochemotherapy (such as dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab [DA-EPOCH-R]; R-CHOP with prophylactic methotrexate [intrathecal or IV]; R-CHOP in combination with a novel agent such as lenalidomide, everolimus, or epratuzumab [in clinical trial settings]; rituximab, cyclophosphamide, etoposide, procarbazine, and prednisone [R-CEPP]; rituximab, cyclophosphamide, doxorubicin, etoposide, cytarabine, bleomycin, vincristine, methotrexate, and prednisone [R-ProMACE-CytaBOM]; rituximab, cyclophosphamide, vincristine, doxorubicin, and high-dose methotrexate, alternating with ifosfamide, etoposide, and cytarabine [R-CODOX-M/IVAC], etc) in the final analysis. Patients with primary mediastinal large B-cell lymphoma or primary central nervous system lymphoma were excluded.
Statistical analysis
The χ2 test was used to compare baseline characteristics between patients with DLBCL alone and patients with concurrent DLBCL and FL or another indolent NHL. Event-free survival (EFS) was defined as the time from diagnosis to disease progression or relapse, unplanned retreatment after initial immunochemotherapy, or death from any cause. OS was defined as the time from diagnosis to death due to any cause. Patients without an event or death were censored at time of last known follow-up. EFS and OS were compared between groups using the Kaplan-Meier method and the log-rank test (not adjusted for other variables), as well as the Cox proportional hazards model. All statistical analyses were done using IBM SPSS Statistics v22.0.
Results
Prevalence and pathology pattern of concurrent indolent NHL in patients with DLBCL
A total of 1521 DLBCL patients in the MER were screened, and 1324 patients with DLBCL alone or concurrent indolent lymphoma treated with R-CHOP or R-CHOP–like immunochemotherapy were included in this study (supplemental Figure 1, available on the Blood Web site). At diagnosis, 1153 patients (87.1%) had DLBCL alone, and 171 patients (12.9%) had concurrent DLBCL and an indolent NHL. The most common indolent component was FL (n = 109; 8.2%). Other histologies included marginal zone lymphoma (MZL) (n = 15; 1.1%), chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) (n = 14;1.1%), lymphoplasmacytic lymphoma (LPL) (n = 2; 0.2%), and B-cell NHL not otherwise specificed (NOS) (n = 31; 2.3%).
The histology pattern of the indolent NHLs that were concurrent with DLBCL is summarized in Table 1. For concurrent DLBCL and FL, 67 patients had DLBCL and FL at the same site (composite histology), 25 patients had DLBCL and FL at different sites (discordant pathology), and 17 patients had both composite and discordant pathology. For concurrent DLBCL and other (or unclassified) indolent NHLs, 8 patients had composite histology, 50 patients had discordant pathology (with the indolent component in the bone marrow in most cases), and 4 patients had both composite and discordant pathology.
Histology and site of the indolent lymphoma that was concurrent with DLBCL
| DLBCL with concurrent: . | FL . | Others* . | MZL . | CLL/SLL . | LPL . | B-cell NHL NOS . |
|---|---|---|---|---|---|---|
| Composite pathology,† n (%) | 67 (61.5) | 8 (12.9) | 5 (33.3) | — | 1 (50.0) | 2 (6.5) |
| Discordant pathology,†n (%) | 25 (22.9) | 50 (80.6) | 9 (60.0) | 11 (78.6) | 1 (50.0) | 29 (93.5) |
| Discordant in tissue | 11 | 2 | 1 | — | 1 | — |
| Discordant in bone marrow | 11 | 48 | 8 | 11 | — | 29 |
| Discordant in both tissue and bone marrow | 3 | — | — | — | — | — |
| Both composite and discordant pathology, n (%) | 17 (15.6) | 4 (6.5) | 1 (6.7) | 3 (21.4) | — | — |
| Discordant in tissue | 2 | — | — | — | — | — |
| Discordant in bone marrow | 14 | 4 | 1 | 3 | — | — |
| Discordant in both tissue and bone marrow | 1 | — | — | — | — | — |
| Total, n | 109 | 62 | 15 | 14 | 2 | 31 |
| DLBCL with concurrent: . | FL . | Others* . | MZL . | CLL/SLL . | LPL . | B-cell NHL NOS . |
|---|---|---|---|---|---|---|
| Composite pathology,† n (%) | 67 (61.5) | 8 (12.9) | 5 (33.3) | — | 1 (50.0) | 2 (6.5) |
| Discordant pathology,†n (%) | 25 (22.9) | 50 (80.6) | 9 (60.0) | 11 (78.6) | 1 (50.0) | 29 (93.5) |
| Discordant in tissue | 11 | 2 | 1 | — | 1 | — |
| Discordant in bone marrow | 11 | 48 | 8 | 11 | — | 29 |
| Discordant in both tissue and bone marrow | 3 | — | — | — | — | — |
| Both composite and discordant pathology, n (%) | 17 (15.6) | 4 (6.5) | 1 (6.7) | 3 (21.4) | — | — |
| Discordant in tissue | 2 | — | — | — | — | — |
| Discordant in bone marrow | 14 | 4 | 1 | 3 | — | — |
| Discordant in both tissue and bone marrow | 1 | — | — | — | — | — |
| Total, n | 109 | 62 | 15 | 14 | 2 | 31 |
—, no case; NOS, not otherwise specified.
All other indolent lymphomas other than FL, including MZL, CLL/SLL, LPL, and B-cell NHL NOS (listed in separate columns as well).
Composite histology means DLBCL and the indolent component were at the same site and discordant histology means DLBCL and the indolent component were at different sites.
Clinical characteristics of DLBCL patients with or without concurrent indolent NHL
Concurrent DLBCL and FL were more common than concurrent DLBCL and other (or unclassified) indolent NHLs, and had mostly composite pathology as opposed to the discordant pathology seen in the latter. Therefore, these cases may be different in clinical characteristics and outcome. As shown in Table 2, compared with patients with DLBCL alone, patients with concurrent DLBCL and FL had fewer elevations in lactate dehydrogenase (P < .01) and lower IPI scores (P < .01), whereas patients with concurrent DLBCL and other indolent NHLs had more advanced stages (P < .01), primarily due to bone marrow involvement, and a trend toward higher IPI (P = .09). Cell of origin (COO) by the Hans algorithm17 was predominantly the germinal center B-cell–like (GCB) for DLBCL that was concurrent with FL (92.9% vs 61.8% for DLBCL alone; P < .01), and was enriched for non-GCB for concurrent DLBCL and other indolent NHLs (52.3% vs 38.2%; P = .06).
Baseline characteristics of patients with DLBCL alone, concurrent DLBCL and FL, and concurrent DLBCL and other indolent NHL
| . | DLBCL alone, n . | % . | Concurrent DLBCL and FL, n . | % . | P, vs DLBCL alone . | Concurrent DLBCL and other indolent NHL, n . | % . | P, vs DLBCL alone . |
|---|---|---|---|---|---|---|---|---|
| Age | .39 | .28 | ||||||
| ≤60 y | 490 | 42.5 | 51 | 46.8 | 22 | 35.5 | ||
| >60 y | 663 | 57.5 | 58 | 53.2 | 40 | 64.5 | ||
| Sex | .10 | .55 | ||||||
| Male | 658 | 57.1 | 71 | 65.1 | 33 | 53.2 | ||
| Female | 495 | 42.9 | 38 | 34.9 | 29 | 46.8 | ||
| ECOG PS | .80 | .51 | ||||||
| <2 | 946 | 82.2 | 89 | 83.2 | 53 | 85.5 | ||
| ≥2 | 205 | 17.8 | 18 | 16.8 | 9 | 14.5 | ||
| Missing | 2 | 2 | ||||||
| Ann Arbor stage | .12 | <.01 | ||||||
| I-II | 441 | 38.2 | 33 | 30.6 | 5 | 8.2 | ||
| III-IV | 712 | 61.8 | 75 | 69.4 | 56 | 91.8 | ||
| Missing | 1 | 1 | ||||||
| LDH | <.01 | .37 | ||||||
| Normal | 443 | 42.3 | 60 | 63.8 | 28 | 48.3 | ||
| Elevated | 604 | 57.7 | 34 | 36.2 | 30 | 51.7 | ||
| Missing | 106 | 15 | 4 | |||||
| Extranodal sites | .38 | .16 | ||||||
| ≤1 | 922 | 80.0 | 91 | 83.5 | 45 | 72.6 | ||
| >1 | 231 | 20.0 | 18 | 16.5 | 17 | 27.4 | ||
| IPI score | <.01 | .09 | ||||||
| 0-1 | 382 | 33.1 | 39 | 35.8 | 11 | 17.7 | ||
| 2 | 317 | 27.5 | 46 | 42.2 | 22 | 35.5 | ||
| 3 | 308 | 26.7 | 15 | 13.8 | 19 | 30.6 | ||
| 4-5 | 146 | 12.7 | 9 | 8.3 | 10 | 16.1 | ||
| Bone marrow involvement | — | — | ||||||
| DLBCL | 156 | 13.5 | 9 | 8.3 | 2 | 3.2 | ||
| Indolent NHL | 0 | 0 | 28 | 25.7 | 52 | 83.9 | ||
| No involvement | 900 | 78.1 | 65 | 59.6 | 7 | 11.3 | ||
| Not done/missing | 97 | 8.4 | 7 | 6.4 | 1 | 1.6 | ||
| Cell of origin | <.01 | .06 | ||||||
| GCB | 490 | 61.8 | 79 | 92.9 | 21 | 47.7 | ||
| Non-GCB | 303 | 38.2 | 6 | 7.1 | 23 | 52.3 | ||
| Missing | 360 | 24 | 18 |
| . | DLBCL alone, n . | % . | Concurrent DLBCL and FL, n . | % . | P, vs DLBCL alone . | Concurrent DLBCL and other indolent NHL, n . | % . | P, vs DLBCL alone . |
|---|---|---|---|---|---|---|---|---|
| Age | .39 | .28 | ||||||
| ≤60 y | 490 | 42.5 | 51 | 46.8 | 22 | 35.5 | ||
| >60 y | 663 | 57.5 | 58 | 53.2 | 40 | 64.5 | ||
| Sex | .10 | .55 | ||||||
| Male | 658 | 57.1 | 71 | 65.1 | 33 | 53.2 | ||
| Female | 495 | 42.9 | 38 | 34.9 | 29 | 46.8 | ||
| ECOG PS | .80 | .51 | ||||||
| <2 | 946 | 82.2 | 89 | 83.2 | 53 | 85.5 | ||
| ≥2 | 205 | 17.8 | 18 | 16.8 | 9 | 14.5 | ||
| Missing | 2 | 2 | ||||||
| Ann Arbor stage | .12 | <.01 | ||||||
| I-II | 441 | 38.2 | 33 | 30.6 | 5 | 8.2 | ||
| III-IV | 712 | 61.8 | 75 | 69.4 | 56 | 91.8 | ||
| Missing | 1 | 1 | ||||||
| LDH | <.01 | .37 | ||||||
| Normal | 443 | 42.3 | 60 | 63.8 | 28 | 48.3 | ||
| Elevated | 604 | 57.7 | 34 | 36.2 | 30 | 51.7 | ||
| Missing | 106 | 15 | 4 | |||||
| Extranodal sites | .38 | .16 | ||||||
| ≤1 | 922 | 80.0 | 91 | 83.5 | 45 | 72.6 | ||
| >1 | 231 | 20.0 | 18 | 16.5 | 17 | 27.4 | ||
| IPI score | <.01 | .09 | ||||||
| 0-1 | 382 | 33.1 | 39 | 35.8 | 11 | 17.7 | ||
| 2 | 317 | 27.5 | 46 | 42.2 | 22 | 35.5 | ||
| 3 | 308 | 26.7 | 15 | 13.8 | 19 | 30.6 | ||
| 4-5 | 146 | 12.7 | 9 | 8.3 | 10 | 16.1 | ||
| Bone marrow involvement | — | — | ||||||
| DLBCL | 156 | 13.5 | 9 | 8.3 | 2 | 3.2 | ||
| Indolent NHL | 0 | 0 | 28 | 25.7 | 52 | 83.9 | ||
| No involvement | 900 | 78.1 | 65 | 59.6 | 7 | 11.3 | ||
| Not done/missing | 97 | 8.4 | 7 | 6.4 | 1 | 1.6 | ||
| Cell of origin | <.01 | .06 | ||||||
| GCB | 490 | 61.8 | 79 | 92.9 | 21 | 47.7 | ||
| Non-GCB | 303 | 38.2 | 6 | 7.1 | 23 | 52.3 | ||
| Missing | 360 | 24 | 18 |
—, not calculated; ECOG PS, Eastern Cooperative Oncology Group performance status; IPI, International Prognostic Index; LDH, lactate dehydrogenase.
Clinical outcome of DLBCL patients with or without concurrent indolent NHL
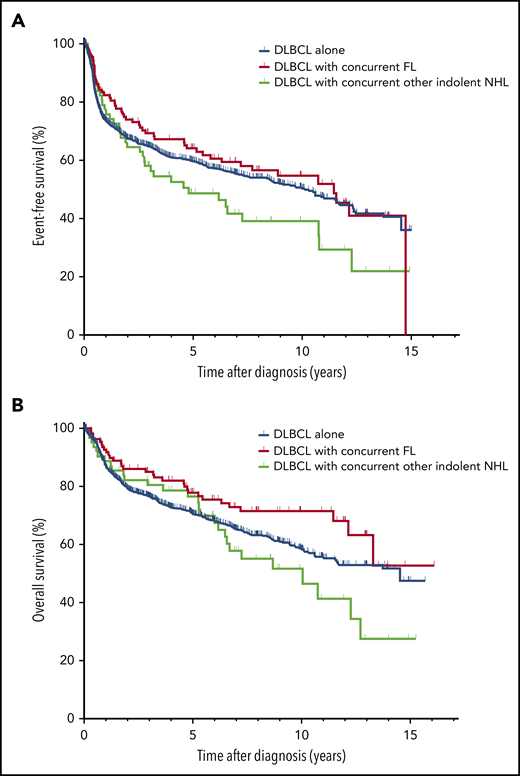
The median follow-up of all living patients was 6 years (range, 0.0-16.1 years). There were a total of 591 events and 444 deaths. As shown in Figure 1, compared with patients with DLBCL alone, patients with concurrent DLBCL and FL had similar EFS (median EFS, 11.5 years [95% confidence interval [CI], 8.0-14.9] vs 10.1 years [95% CI, 8.5-11.7]; P = .47), and a trend of better OS (median OS not reached [95% CI, 12.2 to not applicable (NA)] vs 14.5 [95% CI, 11.6-NA] years; P = .05). In contrast, patients with concurrent DLBCL and other indolent NHLs had a trend of worse EFS (median EFS, 4.8 years [95% CI, 1.2-8.4] vs 10.1 years; P = .09), but similar OS (median OS, 10.0 years [95% CI, 5.8-14.3] vs 14.5 years; P = .33). In patients with concurrent DLBCL and FL or another indolent lymphoma, the site of the indolent component, for example, bone marrow (n = 80) vs other sites (n = 91) did not affect EFS or OS (data not shown). The results were similar in univariate Cox regression analysis (Table 3). After adjusting for IPI, patients with concurrent DLBCL and FL had similar EFS (hazard ratio [HR] = 0.95; 95% CI = 1.71-1.27) and a trend of better OS (HR = 0.75; 95% CI = 0.52-1.09) compared with patients with DLBCL alone, whereas patients with concurrent DLBCL and other indolent NHLs had similar EFS (HR = 1.19; 95% CI = 0.86-1.66) and OS (HR = 1.09; 95% CI = 0.74-1.60) compared with patients with DLBCL alone (Table 3).
Survival in patients with DLBCL or with concurrent DLBCL and FL or other indolent NHL. EFS (A) and OS (B) in patients with DLBCL alone, concurrent DLBCL and FL, or concurrent DLBCL and other indolent NHL.
Survival in patients with DLBCL or with concurrent DLBCL and FL or other indolent NHL. EFS (A) and OS (B) in patients with DLBCL alone, concurrent DLBCL and FL, or concurrent DLBCL and other indolent NHL.
Cox regression analysis of EFS and OS
| . | . | . | Univariate . | Multivariate* . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | No. of events . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| EFS | ||||||||
| DLBCL alone | 1153 | 503 | 1.00 | Reference | .16 | 1.00 | Reference | .54 |
| Concurrent DLBCL and FL | 109 | 50 | 0.90 | 0.67-1.20 | 0.95 | 0.71-1.27 | ||
| Concurrent DLBCL and other indolent NHL | 62 | 38 | 1.33 | 0.96-1.85 | 1.19 | 0.86-1.66 | ||
| DLBCL alone, GCB subtype | 490 | 197 | 1.00 | Reference | .81 | 1.00 | Reference | .99 |
| Concurrent DLBCL and FL | 109 | 50 | 0.96 | 0.71-1.32 | 1.00 | 0.73-1.36 | ||
| OS | ||||||||
| DLBCL alone | 1153 | 385 | 1.00 | Reference | .08 | 1.00 | Reference | .27 |
| Concurrent DLBCL and FL | 109 | 31 | 0.69 | 0.48-1.00 | 0.75 | 0.52-1.09 | ||
| Concurrent DLBCL and other indolent NHL | 62 | 28 | 1.21 | 0.82-1.78 | 1.09 | 0.74-1.60 | ||
| DLBCL alone, GCB subtype | 490 | 142 | 1.00 | Reference | .24 | 1.00 | Reference | .37 |
| Concurrent DLBCL and FL | 109 | 31 | 0.79 | 0.54-1.17 | 0.84 | 0.56-1.23 | ||
| . | . | . | Univariate . | Multivariate* . | ||||
|---|---|---|---|---|---|---|---|---|
| n . | No. of events . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| EFS | ||||||||
| DLBCL alone | 1153 | 503 | 1.00 | Reference | .16 | 1.00 | Reference | .54 |
| Concurrent DLBCL and FL | 109 | 50 | 0.90 | 0.67-1.20 | 0.95 | 0.71-1.27 | ||
| Concurrent DLBCL and other indolent NHL | 62 | 38 | 1.33 | 0.96-1.85 | 1.19 | 0.86-1.66 | ||
| DLBCL alone, GCB subtype | 490 | 197 | 1.00 | Reference | .81 | 1.00 | Reference | .99 |
| Concurrent DLBCL and FL | 109 | 50 | 0.96 | 0.71-1.32 | 1.00 | 0.73-1.36 | ||
| OS | ||||||||
| DLBCL alone | 1153 | 385 | 1.00 | Reference | .08 | 1.00 | Reference | .27 |
| Concurrent DLBCL and FL | 109 | 31 | 0.69 | 0.48-1.00 | 0.75 | 0.52-1.09 | ||
| Concurrent DLBCL and other indolent NHL | 62 | 28 | 1.21 | 0.82-1.78 | 1.09 | 0.74-1.60 | ||
| DLBCL alone, GCB subtype | 490 | 142 | 1.00 | Reference | .24 | 1.00 | Reference | .37 |
| Concurrent DLBCL and FL | 109 | 31 | 0.79 | 0.54-1.17 | 0.84 | 0.56-1.23 | ||
Adjusted for IPI (continuous).
Because the DLBCLs that were concurrent with FL were predominantly the GCB subtype, we compared the outcome of patients with concurrent DLBCL and FL to that of those with GCB DLBCL alone. As shown in Figure 2, these patients had similar EFS (median EFS, 11.5 years [95% CI, 6.3 to NA] vs 10.6 years [95% CI, 8.5-12.7]; P = .81) and OS (median OS not reached for both; P = .24). The results were similar in univariate Cox regression analysis (Table 3). After adjusting for IPI, patients with concurrent DLBCL and FL had nearly identical EFS (HR = 1.00; 95% CI = 0.73-1.36) and OS (HR = 0.84; 95% CI = 0.56-1.23) compared with patients with DLBCL alone (Table 3).
Survival in patients with GCB DLBCL or with concurrent DLBCL and FL. EFS (A) and OS (B) in patients with the GCB subtype of DLBCL and in patients with concurrent DLBCL and FL.
Survival in patients with GCB DLBCL or with concurrent DLBCL and FL. EFS (A) and OS (B) in patients with the GCB subtype of DLBCL and in patients with concurrent DLBCL and FL.
Relapse pattern in patients with concurrent DLBCL and indolent NHL
The relapse patterns in patients with concurrent DLBCL and indolent NHL who had disease relapses during follow-up (27 of 109 with concurrent DLBCL and FL, 22 of 62 with concurrent DLBCL and other indolent NHLs) are characterized in the swimmer plot (Figure 3). Relapses with DLBCL and relapses with an indolent component were both present. In addition, some patients had multiple relapses, especially with the indolent component. Furthermore, relapses beyond 2 to 3 years after initial diagnosis were not uncommon.
Swimmer plot showing relapse patterns in patients with concurrent DLBCL and an indolent lymphoma. For pathology at relapse, indolent lymphoma indicates consistent pathology as initial diagnosis, that is, FL in DLBCL + FL cases or other indolent lymphoma in DLBCL + LG cases. Composite lymphoma also indicates same histology as initial diagnosis. Other lymphoma included a mantle cell lymphoma in a DLBCL + FL case, and a classical Hodgkin lymphoma in a DLBCL + LG case. DLBCL + FL, DLBCL with concurrent FL; DLBCL + LG, DLBCL with concurrent other NHL.
Swimmer plot showing relapse patterns in patients with concurrent DLBCL and an indolent lymphoma. For pathology at relapse, indolent lymphoma indicates consistent pathology as initial diagnosis, that is, FL in DLBCL + FL cases or other indolent lymphoma in DLBCL + LG cases. Composite lymphoma also indicates same histology as initial diagnosis. Other lymphoma included a mantle cell lymphoma in a DLBCL + FL case, and a classical Hodgkin lymphoma in a DLBCL + LG case. DLBCL + FL, DLBCL with concurrent FL; DLBCL + LG, DLBCL with concurrent other NHL.
Discussion
To our knowledge, this is the largest series of DLBCL with concurrent indolent NHL. The strengths of this prospective cohort study include enrollment of consecutive patients, pathology review, and classification by a Mayo Clinic or University of Iowa hematopathologist (allowing for a good estimation of the prevalence of concurrent DLBCL and an indolent NHL), use of standard of care treatment (R-CHOP or R-CHOP–like immunotherapy) for all patients, relatively long follow-up, and large sample size. Limitations include potential biases due to arbitrary choices in biopsy strategies (single vs multiple tissue biopsies, excisional vs core needle biopsy, with or without bone marrow biopsy); use of the Hans algorithm in determining COO (varied concordance with gene-expression profiling18 and limited prognostic role in some studies19,20 ); missing data on COO in some patients; lack of fluorescence in situ hybridization data on MYC, BCL2, BCL6 rearrangements; and lack of standardized evaluation of treatment response (as in a clinical trial).
The majority of the concurrent DLBCL and FL cases had a composite histology (both histology at the same nodal or extranodal site). It is unclear whether these represent coevolvement of independent DLBCL and FL clones or early transformation of previously undiagnosed FL. Molecular studies are required to determine the clonal relationships between the DLBCL and FL components. In contrast, other indolent NHLs that were concurrent with DLBCL were primarily found in the bone marrow. Whether the indolent component in the bone marrow is clonally related to the DLBCL remains unclear and also requires future evaluation.21 Light-chain assessment by either flow cytometry or immunohistochemistry can be helpful in deducing clonality in some cases but the result interpretations are often challenging in DLBCL due to lack of plasmacytic differentiation. Molecular studies such as immunoglobulin gene rearrangement are needed to analyze the clonality of the DLBCL and the indolent component, which would be a future research direction.
In the cases with concurrent DLBCL and FL, the DLBCL component was predominately the GCB subtype. This distribution pattern of COO is consistent with results from prior smaller studies.9,10 In contrast, for DLBCL concurrent with other indolent NHLs, the GCB and the non-GCB subtypes were equally common in our series (thus enriched for non-GCB relative to DLBCL alone cases). One recent study reported that DLBCL with discordant bone marrow involvement was predominantly the GCB subtype,14 although some cases may have had FL in the bone marrow.
The reported outcome of patients with current DLBCL and FL varied in small series. A UK study (1975-2010; n = 59) and a Japanese study (2001-2010; n = 45) reported that patients with concurrent DLBCL and FL had inferior PFS, lymphoma-specific survival, and/or OS compared with those with DLBCL alone (n = 285 and 747, respectively).7,8 However, in a Spanish study (2002-2015), patients with concurrent DLBCL and FL (n = 40) had similar PFS and a trend toward better OS (P=.06) with immunochemotherapy compared with those with DLBCL alone (n = 510).9 In our study, all patients were treated with R-CHOP or R-CHOP–like immunochemotherapy. Those with concurrent DLBCL and FL had similar EFS and a trend of better OS compared with patients with DLBCL alone (P = .05); however, when compared with DLBCL patients with the GCB subtype, they had nearly identical OS. Considering that DLBCL concurrent with FL was predominantly the GCB subtype, it is not surprising that these cases had comparable outcomes to the GCB subtype of DLBCL. Based on results of the Spanish9 and our studies, we recommend that DLBCL patients with concurrent FL at the time of diagnosis be included in clinical trials of newly diagnosed DLBCL. The investigators should be aware, however, that these patients have a treatment outcome similar to GCB DLBCL. Outside of a clinical trial, it is reasonable to treat patients with concurrent DLBCL and FL with standard immunotherapy such as R-CHOP.
The understanding of the natural history of FL transformation has evolved. A prior study from our group has shown that the median OS was superior in patients who were anthracycline naive at transformation compared with those previously treated with anthracycline (5-year OS 66% vs 21%; P < .001), and in patients with transformation >18 months after FL diagnosis (5-year OS of 66% vs 22%; P < .001).22 The National LymphoCare Study reported that patients with concurrent DLBCL and FL at diagnosis had a much improved outcome compared with FL patients who developed transformation later.23 Unlike DLBCL transformed from preexisting FL,2,24,25 high-dose chemotherapy and autologous stem cell transplant may not be needed for patients with concurrent DLBCL and FL at initial diagnosis. In fact, in a Danish study, autologous stem cell transplant did not provide a PFS or OS benefit for selected patients.3
In our study, patients with concurrent DLBCL and other indolent NHLs (primarily in the bone marrow) had similar EFS and OS compared with patients with DLBCL alone after adjusting for IPI. Interpretation of these results should be made with caution as this relatively small cohort was heterogeneous in terms of histology of the indolent component in our study. Although a Korean study reported similar outcomes between DLBCL patients with negative or discordant bone marrow involvement,13 3 other studies in the rituximab era reported that DLBCL patients with discordant bone marrow involvement of an indolent lymphoma had worse PFS but similar OS compared with patients with no bone marrow involvement.11,13,14 An inferior EFS or PFS, even if consistent among studies, is probably not a strong reason to exclude them from DLBCL clinical trials, as DLBCL patients with concordant bone marrow involvement have worse PFS and OS outcomes compared with patients with negative bone marrow11,13,14 but are not usually excluded from clinical trials. However, one may argue that DLBCL patients with a concurrent indolent lymphoma other than FL are a heterogeneous population with unclear disease biology21 and a possibly inferior PFS. Inclusion of these patients in DLBCL clinical trials should be considered based on the goals of the study.
In conclusion, ∼13% of patients with newly diagnosed DLBCL had a concurrent indolent NHL at the time of initial diagnosis, 8% with FL and 5% with other indolent NHLs. DLBCL patients with concurrent FL had predominantly a composite histology and the GCB subtype, with outcomes identical to GCB DLBCL patients. Patients with concurrent other indolent NHLs had predominantly a discordant pathology (primarily in the bone marrow) in advanced stages, with similar EFS and OS compared with those with DLBCL alone after adjusting for IPI.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the National Cancer Institute, National Institutes of Health (P50 CA97274 and U01 CA195568), and the Predolin Foundation.
Authorship
Contribution: Y.W., T.E.W., M.J.M., J.R.C., and G.S.N. designed the study; Y.W., B.K.L., M.J.M., C.A., J.R.C., and G.S.N. collected and analyzed the data; Y.W. wrote the first draft of the manuscript; and all authors reviewed and revised the manuscript and approved the final manuscript.
Conflict-of-interest disclosure: B.K.L. reports a consulting or advisory role for Genentech, Karyopharm, AbbVie, Gilead Sciences, and Celgene, and research funding from Genentech, Pharmacyclics, and Janssen. T.E.W. reports a consulting or advisory role for Incyte, Seattle Genetics, AbbVie, Morphosys, Spectrum, and Immune Design. M.J.M. reports a consulting or advisory role for MorphoSys and research funding from Celgene, NanoString Technologies, and Kite Pharma. A.L.F. reports a consulting or advisory role for Infinity. S.M.A. reports honoraria from WebMD and Research to Practice, and research funding from Bristol-Myers Squibb, Seattle Genetics, Affirmed Therapeutics, Regeneron, Pfizer, LAM Therapeutics, and Trillium Therapeutics. J.R.C. reports a consulting or advisory role for Janssen, and research funding from Celgene and NanoString Technologies. G.S.N. reports a consulting or advisory role for Celgene, MorphoSys, and Genentech, and research funding from Celgene, NanoString Technologies, and MorphoSys. The remaining authors declare no competing financial interests.
Correspondence: Grzegorz S. Nowakowski, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: nowakowski.grzegorz@mayo.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal