The blood cells form a beautiful and elegant system. For a century, hematologists have comfortably understood that each type of blood cell has its own independent function in immunity, hemostasis, or oxygen transport, but in this issue of Blood, Faes and colleagues show that nature is far more efficient than that.1 Faes and colleagues confirm the findings of others that venous fibrin clots entrap red cells. This phenomenon is especially prominent in clots involving sickle erythrocytes (see figure), consistent with the increased rate of venous thromboembolism observed in patients with sickle cell disease.2 They show that fibrin interacts with phosphatidylserine exposed on the senescent sickle red cell membrane. The entrapped sickle red cells make the attached fibrin more resistant to fibrinolysis by tissue plasminogen activator (tPA), exacerbating the prothrombotic effect. This is an unexpected way that sickle red cells modulate the clotting mechanism.
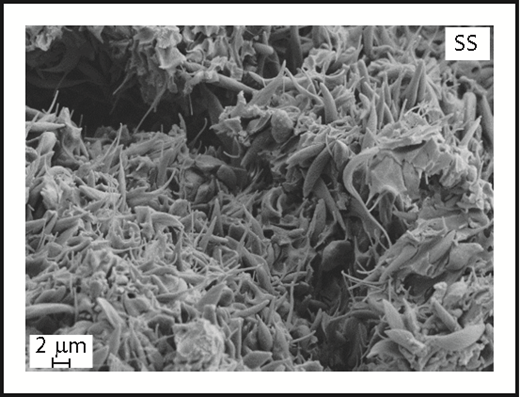
Sickle red cell entrapment in a blood clot. In this scanning electron micrograph, Faes and colleagues show entrapped sickled red cells in an experimentally induced, fatal inferior vena cava blood clot in a sickle cell mouse. The acellular material appears in other images to be a dense fibrin network. Original magnification ×5000. SS, sickle cell. See Figure 1B in the article by Faes et al that begins on page 2529.
Sickle red cell entrapment in a blood clot. In this scanning electron micrograph, Faes and colleagues show entrapped sickled red cells in an experimentally induced, fatal inferior vena cava blood clot in a sickle cell mouse. The acellular material appears in other images to be a dense fibrin network. Original magnification ×5000. SS, sickle cell. See Figure 1B in the article by Faes et al that begins on page 2529.
They contribute to a growing body of evidence that it is naive (and wrong) to believe that leukocytes only fight infection, platelets only provide hemostasis, and erythrocytes only transport oxygen. Many cell types act across traditional functional boundaries. Monocytes express tissue factor that promotes thrombosis.3 Activated neutrophils can promote blood clot formation during inflammatory states, in large part involving formation of neutrophil extracellular traps, physical networks of DNA extruded from adherent neutrophils.4 Likewise, platelets play an important inflammatory role in malaria, autoimmune disease, and atherosclerosis.5 A role for erythrocytes in thrombosis has been proposed in several recent reviews.6 The products of hemolysis are also strongly implicated in promoting pathological vasoconstriction, endothelial adhesiveness, and platelet activation, especially in sickle cell disease.2,7 This is an interesting story, but it gets even better.
The researchers find that blood from sickle cell trait subjects confers tPA resistance intermediate between normal red cells and sickle red cells. What is the relevance of this finding? Several studies have revealed a modest but significantly increased incidence of venous thromboembolism (mainly pulmonary emboli) in the population of sickle cell trait carriers.8 Up until now, there has been no satisfactory mechanism to explain how sickle cell trait red cells might promote increased thrombotic risk. Although sickle cell trait is not a disease, there is some accumulating evidence it is a risk factor for some health outcomes, including pulmonary emboli.9 This new mechanistic finding by Faes and colleagues provides more credibility to the epidemiological findings in sickle cell trait. Not only is sickle cell disease a form of inherited thrombophilia2 but also sickle cell trait may be a mild risk factor for venous thromboembolism.8,9 The implications and incidence of thromboembolism in sickle cell disease are increasingly appreciated, and more investigations are needed to develop best practices in these patients. However, given the high prevalence of sickle cell trait, the population impact could be stunning, even if the relative risk of venous thromboembolism is small. This is certainly an area for future investigation.
Many hematologists might long for a simpler past when neutrophils, platelets, and red cells had distinct and separable functions. The reality is that the complexity of biological systems emerges when scientists develop more sophisticated and open-minded experimental approaches. There is abundant crosstalk and cooperation between the different types of blood cells in the circulation. The blood cells form a beautiful and elegant system.
Conflict-of-interest disclosure: G.J.K. has served as a paid consultant to Novartis, Global Blood Therapeutics, CSL Behring, and Bioverativ and receives research support from Bayer.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal