Abstract
Fostamatinib is a spleen tyrosine kinase inhibitor recently approved for the treatment of chronic immune thrombocytopenia (ITP) in patients without adequate response to at least 1 prior line of therapy. This article reviews fostmatinib’s mechanism of action and its clinical safety and efficacy in 2 industry-sponsored multicenter phase 3 randomized controlled trials in North America, Australia, and Europe (FIT1 and FIT2). Cost comparisons are discussed as well as the role of fostamatinib in relation to other options for chronic ITP.
Introduction
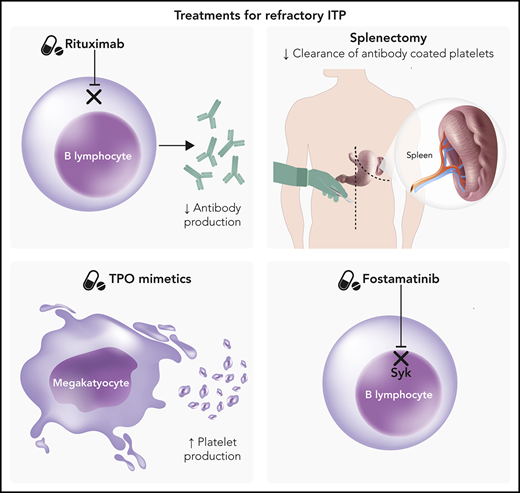
Immune thrombocytopenia purpura (ITP) arises when autoantibodies directed against platelet antigens result in increased clearance of platelets from peripheral circulation.1 In addition, there are data that megakaryocyte dysfunction also plays a role in the pathogenesis of ITP.2 The resulting thrombocytopenia may be asymptomatic or lead to significant bleeding. Chronic ITP is defined as ITP lasting >12 months, and while many patients with chronic ITP do not require treatment, some with severe thrombocytopenia may require sequential lines of therapy or combination therapy in order to maintain a safe platelet count.3 Treatment options for ITP may be broadly classified into treatments that decrease antibody production, treatments that block premature platelet clearance, and treatments that increase platelet production.
First-line therapy for ITP is typically glucocorticoids to decrease antibody production and platelet clearance by macrophages, but many patients are refractory to steroid therapy or unable to be tapered off steroids.4 Second-line therapies include splenectomy, rituximab, and administration of thrombopoietin (TPO) mimetics such as romiplostim and eltrombopag. Each of these therapies requires balance of benefits and risks along with incorporation of patient preferences in order to determine the optimal treatment strategy. For instance, splenectomy has a high response rate resulting in durable remission but also carries a lifelong increased risk of infection (rate ratio 1.9-3.4) as well as thrombosis risk (rate ratio 2.2).5 Rituximab may avoid the surgical risk of splenectomy, but relapses are frequent, with a durable remission rate of only 20% at 5 years, and reactivation of hepatitis B is a concern.6 TPO mimetics provide high response rates but require continuous administration in order to maintain a safe platelet count. While eltrombopag is an oral therapy, romiplostim requires weekly injections, which may be burdensome for some patients.
In April 2018, the US Food and Drug Administration approved the spleen tyrosine kinase (Syk) inhibitor fostamatinib as a novel therapy for the treatment of chronic ITP with an insufficient response to at least 1 prior line of therapy.7
Mechanism of action
Syk is widely expressed in hematopoietic cells, including B cells, T cells, macrophages, and platelets. When Fcγ receptors bind to their ligands, Syk is activated, leading to phosphorylation of immunoreceptor tyrosine-based activation motifs.8 In macrophages, immunoreceptor tyrosine-based activation motif phosphorylation leads to cytoskeletal rearrangement and ultimately to phagocytosis of antibody-covered platelets.7 Syk also plays a role in antibody formation, so inhibition of Syk is an attractive target for ITP therapy.9,10
The Syk inhibitor fostamatinib disodium (R788) is a prodrug, converted by intestinal alkaline phosphatase to the active hydrophobic metabolite R406, which is rapidly absorbed into circulation, with a terminal half-life of ∼15 hours.11,12 R406 is an adenosine triphosphate–competitive inhibitor of the Syk catalytic domain and has a number of downstream effects, including inhibiting FcϵRI signaling in mast cells as well as FcγR and B-cell receptor Syk-dependent signaling.7,8 Early studies of the mechanism of fostamatinib also noted inhibition of Flt3, Jak, and Lck, suggesting the therapeutic benefit of fostamatinib may be multifactorial.7,13 Inhibition of VEGFR2 is believed to cause the increase in blood pressure seen while taking fostamatinib.14 Interestingly, despite decreasing macrophage phagocytosis, fostamatinib does not appear to reduce opsonization of bacteria in preclinical studies, and neutrophil oxidative burst is preserved.8
As Syk plays a role in platelet activation through both the collagen receptor and the integrin αIIbβ3, a theoretical concern is whether fostamatinib might further increase the risk of bleeding in patients already at elevated risk due to thrombocytopenia. Murine studies did not show an increase in bleeding times, and in a single-dose study in healthy human volunteers, fostamatinib had no effect on collagen or adenosine diphosphate–induced platelet aggregation.8
Clinical trials
The efficacy and safety of fostamatinib for treatment of chronic ITP was studied in FIT1 (NCT02076399) and FIT2 (NCT02076412), 2 identical industry-sponsored multicenter phase 3 randomized controlled trials conducted in North America and Australia (FIT1) and in Europe (FIT1 and FIT2).15 Collectively, 150 patients were randomized 2:1 to receive either fostamatinib (n = 101) or placebo (n = 49) twice daily for 24 weeks, and randomization was stratified to balance splenectomy and severity of thrombocytopenia. The median time from diagnosis of ITP to study enrollment was 8.5 years, and ∼75% of the patients carried a diagnosis of ITP for ≥3 years. While only 1 prior line of therapy was required for study inclusion, most subjects had ≥3 therapies (range 1-13), with corticosteroids (93%) or IV immunoglobulin (51%) being the most common. Nearly 50% of subjects previously received TPO mimetics, and approximately one-third of patients had undergone splenectomy, received rituximab, or both.
Efficacy
In the pooled analysis of FIT1 and FIT2, 18% of patients achieved stable response, which the investigators defined as a platelet count >50 × 109/L, without rescue, for at least 4 of 6 weeks between weeks 14 and 24 of treatment. A stable response occurred in only 1 patient in the placebo arm (P = .003). The investigators defined overall response as any platelet count >50 × 109/L within the first 12 weeks. In the pooled analysis, 43% (43 of 101) of those receiving fostamatinib achieved an overall response as compared with 14% (7 of 49) in the placebo arm. While nearly half (46%) of the subjects in the study continued another concomitant ITP medication such as corticosteroids (39.3%), immunoglobulin (3.3%), azathioprine (4.7%), or danazol (1.3%), these were mainly in those who were categorized as nonresponders. Of the stable responders, only 2 subjects were on concurrent steroid therapy. While a subject only needed a platelet count >50 × 109/L at 4 of 6 clinic visits between weeks 14 and 24 to be labeled as a stable response, most of those who responded (77%) achieved this platelet threshold at all 6 clinic visits.
Slightly higher response rates were seen in patients with baseline platelet count <15 × 109/L, with 21% of this subgroup achieving an increase of at least >20 × 109/L to a platelet count >30 × 109/L at week 12, although only 15% maintained this level at week 24. While not powered to evaluate predictors of response, the investigators tentatively identified shorter duration of ITP (<3 years), age <65 years, the presence of antiplatelet antibodies, and a baseline platelet count of 15 to 30 × 109/L as potential predictors worthy of further study.
Results of an extension study, including 44 subjects who originally received placebo in the phase 3 study, were recently published in abstract form. In 27 patients who achieved a stable response, 78% maintained the response at 12 months and 56% maintained the response at 24 months.16
Adverse events
Most patients experienced at least 1 adverse event, and the most common adverse events in the fostamatinib arm included diarrhea (31% overall, 21% mild, 10% moderate, and 1% severe), hypertension (28% overall, 17% mild, 9% moderate, and 2% severe), and nausea (19% overall, 16% mild, 3% moderate, and 0% severe). Diarrhea was graded by the National Cancer Institute Common Technology Criteria for Adverse Events system in which an increase in <4 stools per day over baseline was considered mild (grade 1) and an increase of 4-6 stools per day over baseline was moderate (grade 2). Fostamatinib was continued in grade 1 and 2 diarrhea with administration of loperamide for symptom management. More severe diarrhea required interruption of fostamatinib.
Poorly controlled hypertension, defined as a systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg, was an exclusion criterion regardless of whether the patient was on antihypertensive therapy, although subjects who were treated with antihypertensives after initial screening could participate if their blood pressure decreased to <140/90 mm Hg within 30 days.
In the combined analysis of FIT1 and FIT2, only three of the overall responders stopped fostamatinib due to adverse events. Neutropenia was uncommon (∼1%) and the safety findings were similar to those in a phase 2 study of Syk inhibition in patients with rheumatoid arthritis, where fostamatinib reduced disease activity but was associated with diarrhea, hypertension, and neutropenia.17
Clinical use
Fostamatinib is dosed at 100 mg orally twice daily with dose escalation to 150 mg twice daily if the platelet count remains <50 × 109/L after 4 weeks of therapy. Doses of up to 175 mg twice daily were used in an earlier phase 2 study but were associated with increased toxicity, so the maximum dose evaluated in the phase 3 study was 150 mg twice daily.18 The drug should be discontinued after 12 weeks if the platelet count does not rise to a level that prevents bleeding.
Recommended monitoring requirements while patients are on fostamatinib include monthly monitoring of liver function tests and absolute neutrophil count. The increased risk of overall infection seen in patients on fostamatinib in FIT1 and FIT2 (30% vs 21%) may be secondary to the effect of fostamatinib on both macrophage opsonization and decreased humoral antibody production, but the low rates of severe infection compared with placebo (8% vs 6%) may reflect a preserved neutrophil oxidative burst. The US Food and Drug Administration label does not recommend any specific monitoring or vaccination strategy, but ensuring patients are up-to-date on recommended vaccinations is prudent.
Blood pressure must be checked every 2 weeks until stable, especially if the patient requires concurrent antihypertensive therapy. Animal studies suggest fostamatinib may cause fetal harm, and women of reproductive potential should be counseled regarding effective contraception use. While there are no data regarding lactation, women should be advised not to breastfeed while taking fostamatinib or in the first month after stopping the treatment.
The fostamatinib package insert notes that it is “an inhibitor of the human P-gp efflux transporter in vitro” and that “CYP3A4…is involved in the metabolism of R406,” the active metabolite of fostamatinib. Strong inhibitors of CYP3A4 will increase the R406 concentration, while inducers of CYP3A4 will decrease R406 concentration. Phase 1 clinical studies show an interaction between fostamatinib and both simvastatin and rosuvastatin, with statin AUC almost doubling, which could increase the risk of statin-related adverse events.19 Adjustment of statin doses should be considered if concurrent therapy is necessary, and discontinuation of the statin or fostamatinib may be necessary if statin-specific adverse events arise, depending on which therapy is deemed more important for the patient.19
Current guidelines for ITP recommend the use of corticosteroids as first-line therapy.20 Response rates at 14 days are higher in those treated with dexamethasone (79%) than in those treated with prednisone (59%), although the overall response at 6 months is similar (54% vs 43%, respectively).21 Second-line therapies include splenectomy (response rate 72%)22 and rituximab (initial response rate 62.5%, with median-duration ∼1-year and 5-year sustained response rate of 20%)6,23 for those patients without a response to steroids or those unable to maintain a safe platelet count upon tapering steroids. Many patients, however, will require ongoing therapy. One of the most common subsequent line therapies has been the use of TPO mimetics such as romiplostim and eltrombopag, both with relatively high response rates of 74% and 80%, respectively.24,25 Response rates are higher in those who have previously undergone splenectomy. Fostamatinib offers an attractive option to many as an oral therapy that does not require frequent visits for injection therapy, such as romiplostim, but does require ongoing therapy (Table 1). 4-6,15,26-29 While eltrombopag is also an oral therapy, it has significant dietary restrictions, such as avoiding dairy products for 2 hours before and 4 hours after administration. These dietary restrictions do not appear to be an issue with fostamatinib, although taking the medication with food increases peak serum concentration levels.
Therapies for relapsed or resistant ITP
| Therapy . | Response rate . | Time to response . | Toxicity . | Duration of response . |
|---|---|---|---|---|
| Splenectomy | 80% overall, | 1-24 d | Surgical complications | Approximately 2/3 of patients will require no further therapy |
| 66% stable | Infection (2-3 times baseline risk) | |||
| Thrombosis (∼2 times baseline risk) | ||||
| Rituximab | 60% overall, | 1-8 wk | Hypersensitivity reactions | 20-25% sustained at 5 y, although patients may be retreated |
| 40% stable | Immune suppression | |||
| Hepatitis B reactivation | ||||
| TPO mimetics (eg, romiplostim, eltrombopag) | >80% overall, | 2-3 wk | Rebound thrombocytopenia | Continuous as long as drug is administered |
| 40-50% stable | Thrombosis | In patients who have an initial response, >90% maintain that response at 5 y | ||
| Hepatotoxicity (eltrombopag) | ||||
| Increased marrow reticulin deposition (1.8-7%) | ||||
| Syk inhibitor (fostamatinib) | 43% overall, | 2-8 wk | Diarrhea, nausea | Unknown, but assumed to be continuous as long as drug is administered |
| 18% stable | Hypertension | |||
| Neutropenia |
| Therapy . | Response rate . | Time to response . | Toxicity . | Duration of response . |
|---|---|---|---|---|
| Splenectomy | 80% overall, | 1-24 d | Surgical complications | Approximately 2/3 of patients will require no further therapy |
| 66% stable | Infection (2-3 times baseline risk) | |||
| Thrombosis (∼2 times baseline risk) | ||||
| Rituximab | 60% overall, | 1-8 wk | Hypersensitivity reactions | 20-25% sustained at 5 y, although patients may be retreated |
| 40% stable | Immune suppression | |||
| Hepatitis B reactivation | ||||
| TPO mimetics (eg, romiplostim, eltrombopag) | >80% overall, | 2-3 wk | Rebound thrombocytopenia | Continuous as long as drug is administered |
| 40-50% stable | Thrombosis | In patients who have an initial response, >90% maintain that response at 5 y | ||
| Hepatotoxicity (eltrombopag) | ||||
| Increased marrow reticulin deposition (1.8-7%) | ||||
| Syk inhibitor (fostamatinib) | 43% overall, | 2-8 wk | Diarrhea, nausea | Unknown, but assumed to be continuous as long as drug is administered |
| 18% stable | Hypertension | |||
| Neutropenia |
The low rate of stable response (18%) may reflect the fact that many patients had required multiple prior lines of therapy, suggesting a disease that is inherently more refractory to treatment. Clinical trials of current therapies including rituximab and TPO mimetics were performed when fewer treatment options existed, and the differential response rates compared with fostamatinib may be partially attributed to differences in the population studied in FIT1 and FIT2.
The side effect profile of fostamatinib therapy should not be underestimated. Many patients experienced diarrhea, which was successfully managed with antidiarrheal therapy; however, the change in stool frequency may be bothersome, even with appropriate supportive care. Study subjects required a blood pressure <140/90 mm Hg in order to be eligible for study entry. Patients with preexisting hypertension should be treated to a goal blood pressure of <140/90 mm Hg prior to starting fostamatinib and may require adjustment of antihypertension therapy or cessation of fostamatinib if blood pressure rises during treatment.
Cost considerations
A 1-month supply of fostamatinib carries an average wholesale price (AWP) of US$11 340.1 For comparison, the AWP of a 30-day supply of eltrombopag is $9906 for the 50-mg dose and $14 856 for the 75-mg dose.2 A 250-μg dose of romiplostim, the approximate dose a 70-kg patient would receive weekly at 3 μg/kg, carries an AWP of US$2165.34,3 with a comparable monthly price of US$8661.36. With similar AWPs, differences in efficacy and patient preferences will likely be the major determinants of the cost-effectiveness of fostamatinib in comparison with other available treatments.
Conclusions
Fostamatinib is a novel therapy for the treatment of chronic ITP in patients without an adequate platelet response to at least 1 prior line of therapy, but its low stable response rate of 18% makes it unlikely to replace splenectomy, rituximab, or TPO mimetics as subsequent therapies until direct comparative studies are performed to determine its place in treatment algorithms. For patients who prefer a limited course of treatment with higher durable response rates, splenectomy and rituximab will likely continue to be the preferred second-line therapy. For those patients who prefer to avoid splenectomy and may not be a candidate for rituximab, fostamatinib offers a reasonable oral alternative to romiplostim injections, although eltrombopag is an oral therapy with higher response rates. In our own practice, we consider fostamatinib in patients who have failed to achieve a safe platelet count with other therapies. By addressing the pathophysiology of ITP through a novel mechanism, combination of fostamatinib with other therapies is a potential therapeutic niche for this treatment, but further studies are needed to evaluate the efficacy of this approach before combination therapy with fostamatinib and other therapies becomes routine practice.
Authorship
Contribution: N.T.C. and N.B. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nancy Berliner, Hematology Division, Brigham and Women’s Hospital, 75 Francis St, Boston, MA 02115; e-mail: nberliner@bwh.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal