Abstract
Background. Interferon alpha (rIFNa) has been used as an initial therapy for polycythemia vera (PV) at our institution since the 1980s owing to its efficacy and tolerability at the administered doses(Silver, 2006), and because of emerging evidence that it may alter its natural history(Kiladjian, Giraudier, & Cassinat, 2016). The European Leukemia Net 2018 and other groups included rIFNa in the initial management recommendations of PV. However, there is a need for studies comparing long-term outcomes of patients treated with rIFNa to those treated with standard-of-care, Hydroxyurea (HU) or Phlebotomy only (PHL-O) in the initial setting.
Objectives. The primary objective of this retrospective study compared the overall survival (OS) and the myelofibrosis progression-free survival (MPFS) of patients initially treated with rIFNa to those initially treated with HU or PHL-O. The secondary objective compared the rates of cardiovascular events occurring during treatment with rIFNa and HU at any line of therapy.
Methods. Data extracted from the medical records of PV patients at Weill Cornell Medicine from 1974 until 2018 included date of diagnosis, CV risk factors, CV events, therapies, myelofibrotic progression and survival. Initial therapy was defined as the earliest treatment given for at least one year. Fisher's exact test compared demographic and clinical differences among initial rIFNa, HU, and PHL-O treatment groups and Kaplan-Meier analysis compared OS and MPFS. The risk of cardiovascular events was compared using Cox Proportional Hazards regression analysis.
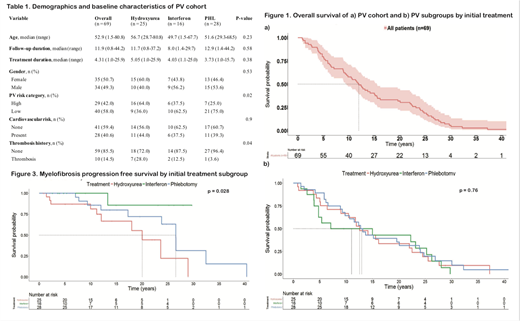
Results. Data collection was completed for 84 of 320 PV patients. Of the 84, 16(19%), 25(30%) and 28(33%) were treated with initial rIFNa, HU and PHL-O respectively, and 15(18%) received other initial therapies. The 15 patients who received other initial therapies were excluded from the analysis. The overall median age at diagnosis was 52.9 years (range 1.5-81) which was not statistically different between the 3 subgroups (rIFNa: 49.7 [1.5-68], HU: 56.7 [29-81], and PHL-O: 51.6 [29-69]). The cohort had 35 (51%) females and the three subgroups were similar in gender distribution (Table 1). The subgroups were also similar in CV risk factors. They differed significantly in PV risk category (p=0.02) and thrombosis history (p=0.04), with the HU subgroup having more high risk patients and more patients with a history of thrombosis (Table1).
The median follow-up duration of the entire cohort was 12 years (range 1-44) and the median initial treatment duration was 4.3 years (range 1-26) (Table1). The median OS of the cohort was 12 years (95% CI: 9.6,15.5) (Figure 1a). The primary outcome of median OS was not significantly different among subgroups and was 15 years for rIFNa (95% CI: 4.8, 28), 12.6 years for HU (95% CI: 10.5, 24.5), and 13 years for PHL-O (95% CI: 9.8, 24.1) (Figure 1b). The MPFS however, was significantly longer with rIFNa compared to HU or PHL, with a median that was not reached within the defined follow-up duration (Figure 2). Older age at diagnosis was associated with significantly increased risk of progression to myelofibrosis (HR 1.05, relative risk per year, p=0.02). After adjusting for age in a Cox model, the relative risk of myelofibrosis progression trended lower when comparing initial rIFNa to HU patients, but did not meet pre-established statistical cutoffs (HR 0.14, 95% CI 0.02-1.17, p=0.07).
The risk of CV events, after adjusting for age, PV risk category, CV risk factors, and thrombosis history, also tended to be lower during rIFNa therapy than during HU therapy, but the difference was not statistically significant (HR 0.23, CI 0.05-1.11, p=0.07).
Conclusion. No significant difference in overall survival was observed between PV patients treated with initial rIFNa compared to initial HU or PHL-O. Initial treatment with rIFNa was associated with a lower risk of myelofibrotic progression. However, this risk was not statistically significant after adjusting for age at diagnosis, which was a predictor of myelofibrosis progression in this sample cohort. Finally, the risk-adjusted incidence of cardiovascular events was lower during IFNa therapy than HU therapy. A larger cohort is needed to validate these findings and accordingly, the data collection and analysis for our total cohort of 320 patients is ongoing.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal