Abstract
Introduction
The international prognostic index (IPI) was a most common prognostic score in patients with aggressive non-Hodgkin lymphoma. Therefore, rituximab improved OS in patients with diffuse large B-cell lymphoma (DLBCL), revised IPI (R-IPI) was suggested. Although, the 5-year OS is higher than 50% in patients of high risk R-IPI. National Comprehensive Cancer Network published an enhanced IPI (NCCN-IPI) for improving risk stratification. However, NCCN-IPI didn't enough evaluate clinical and disease characteristics, for example beta-2-microglobulin (B2MG), cell of origin, albumin, soluble interleukin-2 receptor (sIL-2R). Aim of this study is to evaluate clinical and disease prognostic factor in patients with DLBCL.
Methods
We retrospectively analyzed 647 patients who were newly diagnosed de novo DLBCL and treated with R-CHOP in our hospital from January 2005 to December 2016. We excluded primary central nervous system lymphoma and transformed from low grade B-cell lymphoma. Median follow-up time is 58.5 months (range; 2-160 months.).
We collected data of blood and imaging test before chemotherapy. The patient's baseline clinical and disease characteristics included age, Ann Arbor stage, serum albumin, bulky, B symptoms (defined as recurrent fever, night sweats, or >10% weight loss), B2MG, cell of origin, cluster of differentiation (CD) 5 positive, serum C-reactive protein (CRP), ECOG performance status (PS), ferritin, gender, lactate dehydrogenase (LDH), sIL-2R, monocyte, number and site of involvement extranodal. We evaluate over 60-years old, advance stage (Ann Arbor stage Ⅲ-Ⅳ) and more than PS 2. We defined the cut-off value of B2MG, CRP, ferritin, sIL-2R, monocyte and albumin by using receiver operating characteristic curves. LDH was over the upper limit of normal. Cell of origin was classified by Hans' algorithm. CD 5 was evaluated by immunohistochemistry. The univariate analyses of between factors and OS was used in univariate of log-rank test. And the multivariate analysis was used cox proportional hazards regression analysis.
Results
The patient's median age was 65 years old (range 17-90 years old.). The cut-off value of B2MG is ≧2.23 mg/L, CRP is ≧0.4 mg/dl, ferritin is ≦250 ng/ml, sIL2-R is ≧1000 U/mL, monocyte is ≧608 /μL and serum albumin is ≦3.4 g/dl.
In the result of univariate analysis, age, stage, albumin, B symptoms, B2MG, CD5, CRP, PS, ferritin, LDH, sIL-2R, monocyte, nonGCB, the number of extranodal sites (>1) and the involvement of bonemarrow, liver, lung, muscle, skin and pancreas affected OS. In multivariate analysis, the worse prognostic factors were age (Hazard ratio (HR) 2.13, 95% confidence interval (CI) 1.27-3.57, p<0.01), albumin (HR 1.59, 95% CI 1.01-2.51, =0.047), B2MG (HR 1.86, 95% CI 1.18-2.93, p<0.01), PS (HR 2.01, 95% CI 1.22-3.33, p<0.01), sIL-2R (HR 1.75, 95% CI 1.11-2.76, p=0.016), pancreas (HR 4.43, 95% CI 1.75-11.22, p<0.01) and skin (HR 1.94, 95% CI 1.11-3.39, p=0.021).
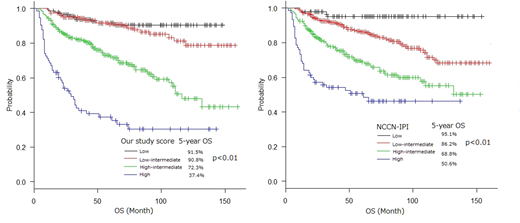
Albumin, B2MG and sIL-2R weren't included prognostic score of IPI, R-IPI and NCCN-IPI. In order to validate we developed prognostic score consisting of 5 factors (age, PS, B2MG, sIL-2R and albumin). And compared with NCCN-IPI in our data. We categorized patients into 4 risk groups: low (0 factor), low-intermediate (1 factor), high-intermediate (2 or 3 factors), high (4 or 5 factors). The low risk of 5-year OS was 91.5%, low-intermediate risk was 86.2%, high-intermediate risk was 72.3% and high risk was 37.4%. The low risk of NCCN-IPI was 95.1%, low-intermediate was 86.2%, high-intermediate was 68.8% and high was 50.6%. The absolute difference in the 5-year OS between low and high risk groups was 54.1% with our study prognostic score compared with 44.5% with NCCN-IPI in our study.
Conclusions
Our study suggest that B2MG, sIL-2R and serum albumin are significant prognostic factors in patients with DLBCL in the rituximab era.
Yokoyama:Chugai pharmaceutical inc, Roche: Other: commissioned work. Mishima:Chugai pharmaceutical inc, Roche: Other: commissioned work. Nishimura:Chugai pharmaceutical inc, Roche: Other: commissioned work. Terui:Novartis pharma: Honoraria; Takeda pharmaceutical: Honoraria; Janssen Pharmaceutical KK: Honoraria; Celgene: Honoraria; Bristol myers Squib: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal