Abstract
Purpose:
Veno-occlusive disease (VOD) is a severe complication of allogenic stem cell transplantation (allo-SCT). The diagnosis of VOD is often difficult and relies on clinical assessment and clinical chemistry. B-mode ultrasound (B-US) and color doppler sonography (CDS) are only of limited additional diagnostic value. We recently observed a patient with VOD, who revealed a pathologic hepatic CEUS pattern (reduced portal venous and parenchymal enhancement). Because CEUS is a measure of perfusion, this suggested that CEUS could potentially be a novel diagnostic tool of VOD (Trenker et al. Bone Marrow Transplantation, 2018). The aim of the present study was to prospectively analyze the hepatic CEUS pattern prior and post allo-SCT and correlate these results with the pre allo-SCT VOD-risk-score, clinical findings suggestive of VOD, as well as with standard B-US and CDS findings.
Patient/ Material/ Methods:
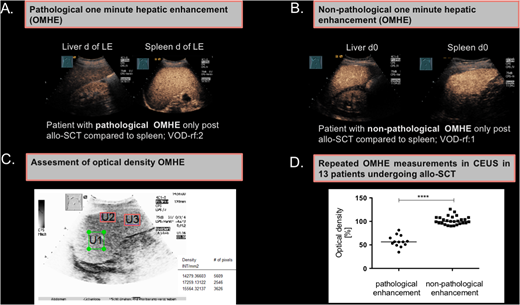
From April to July 2018 13 consecutive patients, who underwent allo-SCT (n=6 AML, n=5 ALL, n=1 MDS, n=1 NHL) and had at least one VOD risk factor (Bu/Cy therapy, TBI, mismatched/haplo donor, prior liver damage, re-allo-SCT) were included. All patients received VOD prophylaxes with 300 IU unfractionated heparin /h. Elevated transaminases, bilirubin, g-GT, ascites, hepatomegaly, splenomegaly, reduced portal flow, weight gain >5 KG, as well as abdominal pain were considered as clinical signs of VOD and assessed prior and during allo-SCT. B-US and CEUS were performed at defined times before and after allo-SCT (day (d) +10 and at leucocyte engraftment (LE). The one-minute hepatic enhancement (OMHE) in CEUS (Fig. 1A, 1B) was standardized to splenic enhancement as in vivo reference by two independent investigators and retrospectively quantitated using optical density (OD) measurements software (QuantityOne) (Fig. 1C). A hypo-OMHE in CEUS was arbitrarily defined as pathological (PE), if the optical density of the liver was less than 90% compared to mean optical density of the spleen.
Results:
Eight of 13 (61.5%) patients had a pathological OMHE in CEUS (1 patient only prior-, 1 patient prior and post, and 6 only post-allo-SCT), suggestive of malperfusion. The median optical density in the pathological OMHE-group was 56.46% (range 34.5-81.4) and thus significantly lower than in the non- pathological group (105.6%, range 90.2-125.9) (p<0.0001) (Fig. 1D). Interestingly, a pathological OD in OMHE was significantly associated with a higher median number of variables indicating clinical signs of VOD (3.25; range 1 to 6 signs) vs. non-pathological OD (0.6; range 0 to 1 signs) (p=0.013). Patients with a pathological OD had also a significantly higher number of VOD-associated B-US and CDS findings when compared to patients with a non-pathological OD (1.5 vs 0.4 signs, p=0.0241). However, the number of pre-treatment VOD-risk factors in the pathological vs. non-pathological OD group was not significantly different.
Conclusion:
Patients undergoing allo-SCT frequently show a pathological hepatic CEUS pattern, which can be quantified by OD measurement and is suggestive of preclinical VOD or yet undefined hepatic pathologies. Further studies to evaluate the clinical significance of a PE with regard to VOD development and risk of GvHD are ongoing.
Burchert:Pfizer: Honoraria; Bayer: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AOP Orphan: Honoraria, Research Funding; Novartis: Research Funding. Görg:Bracco imaging: Other: Bracco imaging supports workshop for contrast enhanced ultrasound for education at the university hospital in Marburg.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal