Abstract
Background:
CXCR4 is a chemokine receptor overexpressed in more than 20 tumor types, including malignant plasma cells. The CXCR4/CXCL12 (SDF-1) axis has been known for many years as a critical regulator of tumor proliferation, cell, as well as migration into and out of the bone marrow. Ulocuplumab (BMS- 936564) is a first in class, fully human IgG4 monoclonal anti-CXCR4 antibody which inhibits the binding of CXCR4 to CXCL12. This study aimed to determine the safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity of Ulocuplumab alone and in combination with lenalidomide plus dexamethasone (Len-Dex), or in combination with bortezomib plus dexamethasone (Bor-Dex) in subjects with relapsed/refractory multiple myeloma.
Patients / Methods:
Patients were eligible for this trial if they were 18 years of age or older with relapsed or relapsed/refractory multiple myeloma after having received at least 2 prior lines of treatment. Patients in whom who both lenalidomide and bortezomib had failed were not excluded from re-treatment with the same regimen. Patients were enrolled at four cancer centers in the U.S. from October 2011 to March 2014. Ulocuplumab (1, 3 and 10 mg/kg) was dose escalated with a 3-plus-3 design with doses of Len-Dex or Bor-Dex to identify maximum tolerated dose (MTD). Ulocuplumab was given weekly in combination with either 25mg lenalidomide on days 1-21 and 40mg oral dexamethasone on days 1, 8, 15, and 22 of the 28-day cycles on Arm A or 1.3 mg/m2 bortezomib on days 1, 4, 8, and 11 and 20mg oral dexamethasone on days 1, 2, 4, 5, 8, 9, 11, and 12 of the 21-day cycles on Arm B since cycle 2. The primary endpoints for this study were dose-limiting toxicities. Other key safety endpoints included incidence of adverse events (AE), AEs leading to discontinuation, SAEs, deaths, and laboratory abnormalities. The efficacy endpoints included overall responses, duration of response, and time to response. Responses were assessed using the IMWG criteria.
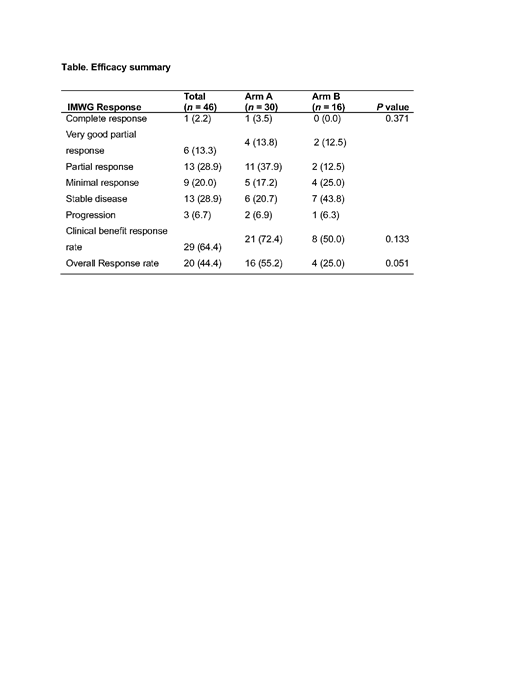
Results:
Forty-six patients were enrolled (median age, 60 years; range, 53-67). The median number of prior therapies was 3 (range, 1-11), with 70.0% of patients having received ≥ 3 lines of treatment. Ulocuplumab was escalated to a maximum of 10 mg/kg without reaching MTD. The most common treatment-related adverse events of any grade were neutropenia (13 patients, 43.3%), diarrhea (10 patients, 33.3%), thrombocytopenia (10 patients, 33.3%), and fatigue (7 patients, 23.3%) in Arm A; and thrombocytopenia (6 patients, 37.5%), fatigue (4 patients, 25.0%) and anemia (4 patients, 25.0%) in Arm B. The overall response rate (≥ partial response) for all subjects in escalation and expansion was 44.4% (20/45). The median time to response was 1.5 months (range 0.4-7.8 months) for Arm A and 1.0 month (range 0.5-3.7 months) for Arm B, respectively. Of note, the combination of Ulocuplumab with Len-Dex showed a high response rate of 55.2% and a clinical benefit rate ( ≥ minimal response) of 72.4%, including patients who have been previously treated with lenalidomide.
Conclusion:
This study shows that the blockade of the CXCR4-CXCL12 axis by Ulocuplumab is safe and has an encouraging response rate of over 50% in the Len-Dex arm of patients with relapsed/refractory myeloma. The distinct mechanisms of action of this antibody, as well as its non- cross resistance with currently approved approaches, make it a new class of anti-myeloma drug that warrants further exploration and evaluation in future clinical trials.
Ghobrial:Takeda: Consultancy; Celgene: Consultancy; BMS: Consultancy; Janssen: Consultancy. Richardson:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees. Anderson:Celgene: Consultancy; Takeda Millennium: Consultancy; Bristol Myers Squibb: Consultancy; Gilead: Membership on an entity's Board of Directors or advisory committees; Oncopep: Equity Ownership; C4 Therapeutics: Equity Ownership. Becker:GlycoMimetics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal