Abstract
BACKGROUND: Multiple myeloma patients who receive immunomodulatory drugs (IMiDs: lenalidomide, thalidomide, and pomalidomide) have an increased risk of developing venous thromboembolism (VTE). Guidelines for thromboprophylaxis are based on additional patient and disease characteristics. We describe our single-institution experience with VTE prophylaxis and an intervention to improve VTE risk assessment and prophylaxis.
METHODS: A retrospective review using an internal patient database assessed VTE in multiple myeloma patients being treated with IMiDs from 2000-2016. VTE risk factors for each patient were assessed to determine alignment with thromboprophylaxis guidelines.
A Quality Improvement (QI) phase from April 1, 2017 to December 31, 2017 added pharmacy oversight to perform an independent VTE risk assessment. Every patient started on an IMiD during this period underwent a separate VTE risk assessment by a pharmacist or hematologist. Each patient was categorized as high or low VTE risk based on NCCN guidelines. The results and recommendations for VTE prophylaxis were given to the myeloma provider.
Results: In the initial retrospective review, 107 patients were identified who developed VTE during treatment of multiple myeloma with an IMiD despite thromboprophylaxis in 91 patients (85% of total; 78% on aspirin). The most common VTE risk factors per NCCN guidelines included cardiac disease (n=70), obesity (n=32), chronic kidney disease (n=27), and prior history of VTE (n=18). Eight patients received anticoagulant-based thromboprophylaxis.
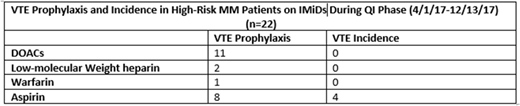
In the QI phase, 39 multiple myeloma patients were started on IMiDs. The risk assessment classified 17 as low-risk and 22 as high-risk. Of the high-risk patients, 14 (64%) were placed on an anticoagulant for thromboprophylaxis. Eleven (79%) of the anticoagulants used were direct oral anticoagulants (DOACs), 2 (14%) were a low-molecular weight heparin, one (7%) warfarin.
The number of thromboembolic events that occurred were 6 (15%): 4 were high-risk on aspirin and 2 were low-risk on aspirin. The 2 low-risk patients who developed VTE had additional provoking factors (active infection, central line placement, smoking, a long driving trip).
Eight high-risk patients were given aspirin. Out of the 8, 3 patients developed VTE and were then switched to anticoagulation. One high-risk patient received aspirin because of moderate thrombocytopenia and subsequently developed a VTE. No patients on anticoagulation developed a VTE. The number of complications attributed to thromboprophylaxis were 2 (5%). Two minor bleeding events occurred in patients who were on DOACs (1 epistaxis and 1 grade 1 GI bleed). Both patients continued DOAC anticoagulation after the event resolved.
Conclusions: This two-phase QI study showed that multiple myeloma patients at high risk for VTE benefit from guideline-based thromboprophylaxis facilitated through a pharmacy-based system. DOAC's ease of use offer patients and providers an agreeable option that may improve compliance of VTE guidelines. However, prospective studies with DOACs in multiple myeloma are urgently needed to support this.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal