Abstract
Introduction: Venetoclax is a potent BCL-2 inhibitor that is approved as monotherapy and in combination with rituximab for patients with relapsed or refractory (R/R) chronic lymphocytic leukemia (CLL) in the United States, and as a monotherapy for patients with 17p deletion or TP53 mutation in the European Union and other countries. Here we report the long-term efficacy and safety of the combination of venetoclax and rituximab from this phase 1b study with a median follow up of >4 years, two years beyond when initially published.
Methods: Patients received daily venetoclax (200 - 600mg) and 6 - 9 doses of rituximab over 6 months, then venetoclax monotherapy.(NCT01682616; Seymour, et al. Lancet Oncol. 2017; 18(2):230-240.) Minimal residual disease (MRD) was assessed in bone marrow (BM) using ≥4-color flow cytometry (min sensitivity: 0.01%). As amended in specific protocol versions, patients who achieved complete remission (CR) or BM MRD-negativity regardless of CR status (collectively considered as deep responses) could stop venetoclax and remain on study, retreating with venetoclax +/- rituximab at clinical disease progression (iwCLL criteria).
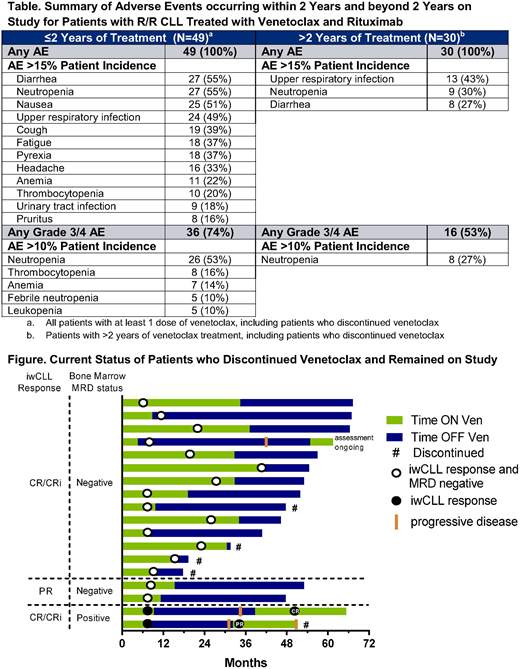
Results: Forty-nine patients, with a median 2 (range: 1 - 5) prior therapies, were enrolled. As of June 4, 2018 (N=49), the median time on study was 4.1 years (range: 0 - 5.8) and the median time on venetoclax was 2.5 years (range: 0 - 5.8). The overall response rate (N=49) was 86%, CR rate was 51%, and BM MRD-negativity rate was 61%. The 48-month estimates (N=49) for overall survival was 89% (95% CI: 75%, 95%), progression free survival was 61% (95% CI: 45%, 74%), and duration of response was 64% (95% CI: 46%, 77%) (for patients with BM MRD-negativity, 88% [95% CI: 66%, 96%]). The adverse events of any grade occurring in >15% of patients beyond 2 years of treatment were upper respiratory infection, neutropenia, and diarrhea (Table).
Disease progression occurred in 17 patients, 14 while on continuous therapy: 5 with Richter transformation at a median of 5 months (range 1 - 8), 9 with CLL progression at a median of 29 months (range: 12 - 55). Three patients (2 MRD-positive CR and 1 MRD-negative CR with CLL cells detectable below the threshold of 10-4) progressed after stopping venetoclax (asymptomatic progression at 23, 24 and 38 months off venetoclax). All three have been re-treated with venetoclax plus rituximab, resulting in CR in one patient (duration of ongoing subsequent CR, 17 months), one partial response (PR) followed by progressive disease 18 months later, and results are pending for the third patient.
Fifteen additional patients stopped venetoclax per protocol following the achievement of a deep response (median duration [range] of treatment 19 months (range: 8 - 40);13 MRD-negative CR, 2 MRD-negative PR) (Figure). Four have discontinued the study (withdrew consent [n=4]) and 11 remain on study in long term follow up with a median duration of ongoing response of 49 months (range: 32 - 59), and a median of 33 months (range: 12 - 58) off venetoclax to date.
Twelve patients who have received continuous venetoclax and remain on study, remain free of disease progression: 8 in CR (7 MRD-negative, 1 MRD-positive) and 4 in MRD-negative PR. Median duration of response on therapy is 51 months (range: 44 - 66). The 4 patients with PR do not meet CR criteria because of lack of BM biopsy results (n=2) and residual adenopathy (n=2; max node dimension; 17 and 23 mm).
The other 5 patients have discontinued because of withdrawal of consent (n=2; 1 without response, 1 MRD-positive PR) and adverse events (n=3; tumor lysis syndrome, worsening peripheral neuropathy, both without response, and fatal ischemic heart disease considered unrelated to therapy in MRD-negative CR [n=1]).
Conclusions: Venetoclax with rituximab induces deep and durable responses in patients with previously treated CLL, with 51% achieving CR and 61% achieving BM MRD-negativity. Those with a deep response can discontinue venetoclax and maintain prolonged treatment-free remission of more than three years, especially if MRD-negative.
Brander:Pharmacyclics, an AbbVie Company: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Genentech: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Teva: Consultancy, Honoraria; BeiGene: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; TG Therapeutics: Consultancy, Honoraria, Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; Novartis: Consultancy, Other: DSMB; Acerta: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding; DTRM: Other: Institutional research funding for non investigator initiated clinical trial, Research Funding. Seymour:Celgene: Consultancy; AbbVie: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Research Funding. Ma:Pharmacyclics: Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Acerta: Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. Anderson:Genentech: Research Funding; AbbVie, Inc: Research Funding; Walter and Eliza Hall: Employment, Patents & Royalties. Choi:Genentech: Speakers Bureau; AbbVie, Inc: Consultancy, Speakers Bureau; Rigel: Consultancy; Pharmacyclics: Consultancy, Research Funding, Speakers Bureau; Gilead: Speakers Bureau. Kipps:Genentech Inc: Consultancy, Research Funding; Celgene: Consultancy; F. Hoffmann-La Roche Ltd: Consultancy, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Verastem: Membership on an entity's Board of Directors or advisory committees. Humphrey:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Masud:AbbVie, Inc: Employment, Equity Ownership. Nandam:AbbVie, Inc: Employment, Equity Ownership. Kim:Abbvie: Employment, Equity Ownership. Verdugo:AbbVie, Inc: Employment, Equity Ownership. Roberts:AbbVie: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Walter and Eliza Hall: Employment, Patents & Royalties: Employee of Walter and Eliza Hall Institute of Medical Research which receives milestone and royalty payments related to venetoclax.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal