Abstract
Introduction: We have previously reported that treatment of patients with relapsed or refractory indolent B-cell lymphoma with the pan-class I phosphatidylinositol 3-kinase (PI3K) inhibitor copanlisib resulted in durable responses with a manageable safety profile (Dreyling et al., J Clin Oncol 35:3898-3905, 2017). The objective response rate was 59%. In contrast with oral PI3K inhibitors, there was also a low incidence of severe adverse events, such as pneumonitis and colitis with copanlisib, possibly due to intravenous dosing and intermittent dose schedule. Because late-onset severe toxicities have been reported with chronic use of some oral PI3K inhibitors, we conducted a 2-year follow-up of the efficacy and safety from this study.
Methods: Patients with histologically confirmed indolent B-cell non-Hodgkin lymphoma (4 subtypes: follicular lymphoma (FL), marginal zone (MZL), small lymphocytic and lymphoplasmacytoid / Waldenstrӧm macroglobulinemia) and relapsed after, or refractory to, ≥2 prior lines of treatment were eligible. Previous treatment had to include rituximab and an alkylating agent or regimen. Copanlisib was administered at a fixed dose of 60 mg via 1-hour I.V. infusion on days 1, 8 and 15 of a 28-day cycle. Treatment continued until progression or unacceptable toxicity. The primary efficacy endpoint was objective response rate (ORR) after ≥4 cycles as assessed per independent radiologic review (Cheson et al., JCO 20:579, 2007). Secondary efficacy endpoints included duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Adverse events were reported using MedDRA (version 19.1). The last patient was enrolled in February 2016. The initial data cut-off was June 2016 and the long-term follow up is based on a February 2018 data cut-off.
Results: A total of 142 patients were treated; the predominant histologies were FL (n=104) and MZL (n=23). The median age was 63 years (range 25-82). Median number of prior lines of treatment was 3 (range 2-9), with 61% being refractory to the last regimen.
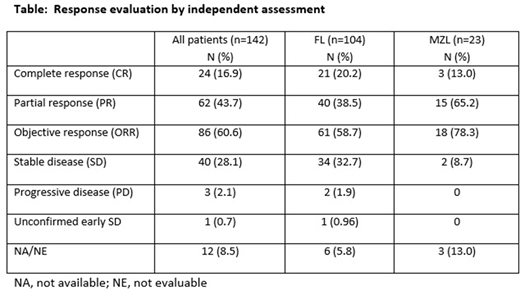
As of February 2018, the median duration of treatment was 6.0 months (range 0.2-44). The ORR was 61% (Table). CRs were seen in 24 patients (17%), 21 in the FL subset (20.2%) and 3 in the MZL group. The 24 CRs represent an increase of 7 compared to the original primary analysis. Median DOR was 14.1 months (95% CI 8.3; 22.3). With 72 events, the median PFS was 12.5 months (95% CI 9.0; 18.4) and with a total of 52 events the median OS was 42.6 months (95% CI 36.5; not reached).
At the data cut-off, 11 patients (9 FL, 2 MZL) remained on treatment (range 24.8-43.9 months). A total of 38 patients (26.8%) had discontinued due an adverse event (AE) not associated with disease progression. Treatment-related AEs leading to dose reductions or dose interruptions were recorded in 36 (25.4%) and 72 (50.7%) patients, respectively. With a median safety follow up of 6.7 months, the most common treatment-emergent AEs (all-grade/grade 3/grade 4) were transient hyperglycemia (50.0%/33.1%/7%) and transient hypertension (29.6%/23.9% G3). Other AEs of interest included neutropenia (28.9%/9.2%/14.8%), diarrhea (35.2%/8.5% G3), pneumonitis (6.3%/1.4% G3), and colitis (one patient with G4). Serious AEs (SAEs) were reported in 55.6% of patients, with 31.7% G3 and 12% G4. (Corresponding SAEs from June 2016 were 50% all-grade, 27.5% G3 and 10.6% G4.) There were no new reports of G5 AEs .
Conclusions: Analysis of the 2-year follow up of patients with relapsed or refractory indolent B-cell lymphoma treated with copanlisib demonstrated a deepening of the responses with a conversion of 7 patients from PR to CR, durable responses and manageable AEs. Moreover, the benefit/risk ratio for copanlisib treatment remains favorable with no evidence of worsening of AEs for patients treated long-term.
Dreyling:Mundipharma: Consultancy, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Acerta: Consultancy; Gilead: Consultancy, Honoraria; Bayer: Consultancy, Honoraria; Sandoz: Consultancy. Leppä:Celgene: Consultancy; Janssen: Consultancy, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Research Funding; Bayer: Research Funding. Follows:Janssen: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Lenz:Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Research Funding; Roche: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding; Janssen: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding, Speakers Bureau; Gilead: Consultancy, Honoraria; Celgene Corp.: Consultancy, Honoraria, Other: Travel, Accomodations, Expenses, Research Funding, Speakers Bureau. Demeter:Amgen: Consultancy; Angelini: Consultancy; Novartis: Consultancy; Roche: Consultancy; Pfizer: Consultancy; BMS: Consultancy; Aramis Pharma: Consultancy. Özcan:MSD: Research Funding; Archigen: Research Funding; MSD: Other: travel support, Research Funding; Novartis: Research Funding; Takeda: Honoraria, Other: Travel payment, Research Funding; Abbvie: Other: Travel payment; Bayer: Research Funding; Celgene: Other: Travel support, Research Funding; Janssen: Other: Travel Support, Research Funding; BMS: Honoraria; Jazz: Other; Jazz: Other: Travel support; Roche: Honoraria, Research Funding. Morschhauser:Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy; Janssen: Other: Scientific Lectures; Roche: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees. Stevens:Bayer: Consultancy, Membership on an entity's Board of Directors or advisory committees. Rodrigues:Bayer: Employment. Hiemeyer:Bayer: Employment. Wang:Syneos Health: Employment. Garcia-Vargas:Bayer: Employment. Childs:Bayer: Employment. Zinzani:Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; SERVIER: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Honoraria, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; PFIZER: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Speakers Bureau; TG Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal