Abstract
Introduction: Complete remission (CR) is an important endpoint after cytarabine plus anthracycline [7+3] induction therapy in Acute Myelogenous Leukemia (AML). Even though CR is observed in about 50-90% of unselected European Leukemia Network 2017 (ELN-2017) patients (pt), factors such as age >65 years, complex karyotype, and adverse mutations (RUNX1, ASLX1, TP53, FLT3 ITD high, KMT2A, secondary AML), leads to inadequate blast eradication. Early response to induction is a predictor of subsequent complete remission (CR). Bone marrow day 14 [D14] inform pt with early inadequate leukemia eradication [blast >10%] who are suitable for re-induction, a strategy that seeks to facilitate conversion to CR or CR with incomplete hematologic recovery (CRi), secure potential allogenic transplantation and reproduce superior outcomes. In this study, we investigated the clinical outcome of an unselected ELN-2017 AML cohort with inadequate D14 marrow response. From the subgroup of patients exhibiting sub-optimal response (SOR), we examined the odds and predictors for subsequent achievement of CR/CRi for those patients who did not receiving immediate re-induction therapy. Additionally, we evaluated the effect of subsequent CR/CRi achievement on survival.
Methods: With prior IRB approval, 160 AML pt diagnosed with AML from 1995 to 2017 within Baylor College of Medicine institutions were evaluated. Kaplan-Meier method was used to estimate overall survival (OS) among pt achieving D14 <> 10% blast in an unselected ELN-2017 AML cohort and pt exhibiting >10% blast in D14 marrow with and without CR/CRi. Logistic and cox regression analysis in SOR pt [1] attaining subsequent CR/CRi and [2] OS, respectively, was performed to investigate multiple independent variables with predictive value for the 2 above outcomes.
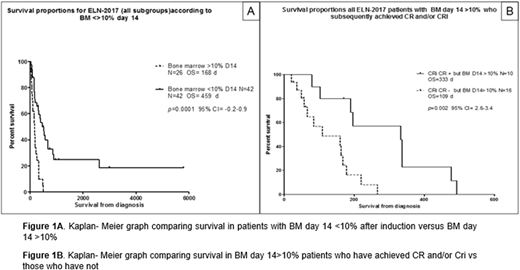
Results: 68/160 (42.5%) of pt had available D14 [early assessment] and sequential day 30 marrow for CR/CRi evaluation. Among 68 unselected ELN-2017 AML pt with D14 marrow for CR/CRi assessment, 42/68 (61.7%) and 26/68 (38.2%) had D14 marrow blasts < and > 10%. Median age was 57 y (range 27-73) and 59 y (range 24-89), respectively, p= 0.74. OS was 459 d vs 169 d in pt with D14 marrow <10% and >10%, at day 14 (p=0.001 95% CI 0.2-0.9) [Fig 1A]. CR/CRi was observed in 36/42 (90%) and 10/26 (38.7%) of pt with D14 marrow <10% vs >10%, respectively, p=0.0005. After controlling for traditional high-risk factors including WBC, age, platelet count, RDW, de novo v secondary AML, only ELN-2017 classification [fav vs unfav and intermediate vs unfav, p= 0.0026 and p=0.01] retained impact on survival. In pt with SOR, we performed second analysis to investigate survival among pt with and without subsequent CR/CRi achievement who did not receive re-induction [Fig 1B]. 16/26 (62.5%) of pt with SOR failed to achieved CR/CRi. OS was 333 d vs 109 d for pt with CR/CRi vs those without CR/CRi [p=0.002, 95% CI 2.6-3.4]. Logistic regression identified in pt with CR/CRi vs those without CR/CRi that: [a] age [63.1 vs 43.6 y-p=0.001]; [b] lower platelet count [47.1 vs 83.1 K/uL-p=0.03]; [c] higher absolute monocyte count (AMC) [3.7 vs 0.41 K/uL-p=0.04]; [d] increased RDW [18.3 vs 14.7-p=0.004] and [e] high BMI [31 vs 24.1-p=0.0003] were significantly associated with failure to achieve CR/CRi. Typical complex karyotype and initial marrow blast % were not associated with subsequent CR/CRi achievement. However, in pt with SOR, lack of high-risk mutations [P53, RUNX, FLT3-ITD, U2AF1] was significant associated with CR/CRi, [40% v 62.5%, p= 0.0004]. Cox proportional regression model showed significant impact on survival for high-risk mutations and higher BMI in survival.
Conclusion: In our retrospective study, despite 38.7% of patients with detectable D14 residual leukemia achieved CR/CRi without re-induction, failure to attain CR/CRi was frequently observed after SOR. Advanced age, lower platelet count, higher AMC, RDW and BMI predict failure to achieve CR/CRi status in patients exhibiting initial SOR. Lack of high-risk mutation was a strong predictor for CR/CRi achievement. Our study is novel by suggesting that a combination of pre-induction and "early post-induction" variables facilitate recognition of high-risk AML subgroups requiring re-induction or alternative novel therapy via clinical trials.
Yellapragada:Takeda: Research Funding; Novartis: Employment; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal