Abstract
Introduction: Patients with acute myeloid leukemia (AML) undergoing consolidation chemotherapy with high dose cytarabine (HiDAC) are often placed on prophylactic antimicrobials. This practice is largely based on the defined benefit of antimicrobial prophylaxis for AML patients during induction chemotherapy. Recent concerns regarding antimicrobial prophylaxis include increased risk of Clostridium difficile colitis, antimicrobial toxicities, and the potential for fostering multidrug-resistant pathogens. These emerging concerns coupled with the relatively shorter duration of neutropenia for AML patients with consolidative chemotherapy raised the question of whether antimicrobial prophylaxis in this setting is necessary. Therefore we examined the role of antimicrobial prophylaxis in HiDAC consolidation within our institution.
Methods: This retrospective review included all adult patients who received HiDAC consolidation chemotherapy between January 2007 and March 2018. HiDAC was defined as a dose of 1g/m2 or greater. In patients receiving more than one cycle of HiDAC, data from each cycle was included. Patients prescribed antibiotics, antiviral medications, or antifungal medications at the time of discharge following HiDAC were considered to have received prophylactic antimicrobials. Several baseline patient characteristics were documented including age, sex, Charlson Comorbidity Index, and ECOG PS. The primary endpoint was incidence of febrile neutropenia (FN), while secondary endpoints consisted of hospitalizations for any cause as well as incidence of bacteremia, invasive fungal infection, Clostridium difficile colitis, and death from infection. Patients admitted within one month following HiDAC consolidation were considered to have been hospitalized following therapy. Data was then analyzed based on whether or not patients received antibiotic and antimicrobial prophylaxis. Patients less than 60 and 60 years of age an older where also analyzed separately regarding the use of prophylactic antibiotics to determine if older patients benefited from antibiotic prophylaxis.
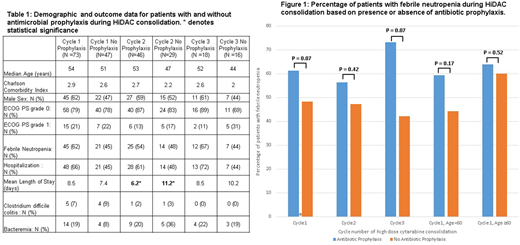
Results: One hundred twenty patients met inclusion criteria, and data from two hundred thirty-three cycles of HiDAC was analyzed. No significant demographic differences were detected amongst patients who did and did not receive antimicrobial prophylaxis (Table 1). There was no difference in incidence of FN or the number of hospitalizations for each cycle of HiDAC consolidation amongst the prophylaxis and no prophylaxis cohorts. The only statistically significant difference between groups was that in cycle 2 the mean length of hospital stay following HiDAC consolidation was less (p=0.02) in patients prescribed prophylactic antimicrobials. The rates of bacteremia and Clostridium difficile colitis were also similar across both groups in all three cycles of HiDAC consolidation (Table 1). In the setting of HiDAC consolidation, only one patient was found to have an invasive fungal infection and zero patients died as a result of infection. When only antibiotic prophylaxis was analyzed there was likewise no difference in the rates of FN across all 3 cycles of HiDAC nor was there a difference in incidence of FN for patients < 60 and patients ≥ 60 (Figure 1). Furthermore, there was no difference in demographics or other outcomes amongst the antibiotic prophylaxis and no prophylaxis cohorts (data not shown).
Discussion: Neither antibiotic nor antimicrobial prophylaxis during consolidation chemotherapy with HiDAC reduced the incidence of FN or frequency of hospitalizations. Additionally, there was no difference in the rates of bacteremia, Clostridium difficile colitis, or invasive fungal infections between the two groups. Importantly, no patients in either cohort died from infectious complications following HiDAC consolidation. In patients with acute myeloid leukemia undergoing HiDAC consolidation, the role of prophylactic antimicrobials remains unclear. Prophylactic antimicrobials may not be necessary for all patients consolidated with HiDAC due to a potentially shorter course of neutropenia when compared to patients with AML undergoing induction chemotherapy, especially amongst younger AML patients. The use of antimicrobial prophylaxis should take into context patient and institutional factors.
Reagan:Pfizer: Research Funding; Takeda Oncology: Research Funding; Alexion: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal