In this issue of Blood, Popescu et al report that anthrax-derived peptidoglycan (PGN) interacts with and supports the activation of coagulation proteins, drives tissue factor (TF) expression, and contributes to systemic disseminated intravascular coagulation (DIC).1
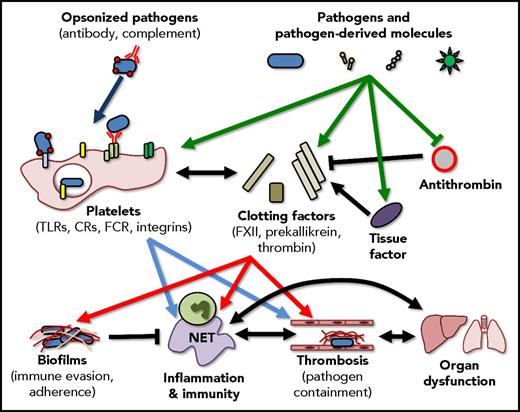
Interactions between pathogens, the immune system, and coagulation are multifaceted and overlapping. Ideally, pathogens and pathogen-derived molecules activate coagulation factors and platelets resulting in the enhancement of the host immune response and the generation of localized thrombi that limit pathogen dissemination. These responses, however, also have the capacity to trigger disseminated coagulopathies and the generation of biofilms, complex structures that shield the pathogen from the immune system, promote bacterial adhesion, and support the persistence of infection. Importantly, these pathways do not exist in isolation, activation of the immune response can further drive coagulation, and events such as thrombin generation can further potentiate the inflammatory response. CR, complement receptor; FCR, Fc receptor; NET, neutrophil extracellular trap.
Interactions between pathogens, the immune system, and coagulation are multifaceted and overlapping. Ideally, pathogens and pathogen-derived molecules activate coagulation factors and platelets resulting in the enhancement of the host immune response and the generation of localized thrombi that limit pathogen dissemination. These responses, however, also have the capacity to trigger disseminated coagulopathies and the generation of biofilms, complex structures that shield the pathogen from the immune system, promote bacterial adhesion, and support the persistence of infection. Importantly, these pathways do not exist in isolation, activation of the immune response can further drive coagulation, and events such as thrombin generation can further potentiate the inflammatory response. CR, complement receptor; FCR, Fc receptor; NET, neutrophil extracellular trap.
Prosescu et al provide clear evidence that PGN directly associates with factor XII (FXII) and prekallikrein, key components of the contact-dependent clotting pathway. Further, this binding appears to enhance the activity of FXIIa and kallikrein and protects FXIIa from inhibition by antithrombin. Interestingly, PGN also induced de novo TF expression by human monocytes in vitro, and by nonhuman primate (NHP) monocytes in vivo, resulting in a parallel activation of the extrinsic clotting pathway. PGN activation of the contact-dependent and the extrinsic clotting pathways resulted in the activation of platelets, the generation of a consumptive coagulopathy, thrombosis, increased vascular permeability, and multiple organ failure in NHP. This work clearly demonstrates the ability of pathogen-derived molecules (PGN) to directly interact with and modulate the activation of coagulation factors, independent of their interactions with the innate or adaptive immune systems (ie, complement, antibody opsonization, Toll-like receptors [TLRs]), and highlights the intimate and overlapping activities of inflammation, immunity, and coagulation.
Importantly, this work involves the study of NHPs, and these findings perhaps best represent the situation in human patients during gram-positive bacteremia and sepsis. Previous studies in rodent models have highlighted key differences present between mice and humans with regard to studying infection-induced coagulation, including differential expression of protease-activated receptors2 on rodent and human platelets and endothelium, the presence of FcγRIIa on human platelets but not mouse platelets,3 and significant differences in the cytokine response to both pathogen-derived molecules and to actual infection. In the current study, the use of NHPs provides convincing evidence that pathogens directly interact with and regulate the activation of the host coagulation response.
The current study by Popescu et al continues to build on, and add to our understanding of the interrelationship between infection, inflammation, and coagulation (see figure). It has been known for some time that pathogens (and pathogen-derived molecules) are able to stimulate the immune system through pattern-recognition receptors (PRRs) such as the TLRs,4 through complement receptors and even through direct interaction with molecules such as integrins,3 leading to the initiation of inflammation and the downstream initiation of coagulation.5 Additionally, it has been previously shown that toxins from coagulase-positive bacteria are able to directly initiate thrombin activation and fibrin generation.6 Popescu et al add to our understanding by showing that a single bacteria-derived molecule, PGN, is able to directly interact with multiple components of the contact-dependent coagulation pathway, protect FXIIa from inhibition by antithrombin, and induce the generation of TF, the key initiator of the extrinsic coagulation pathway. Interestingly, this work nicely parallels other emerging research that demonstrates the ability of α toxin molecules released by Staphylococcus aureus to directly activate platelets, again leading to platelet aggregation and subsequent tissue damage7 suggesting a common theme of direct activation of coagulation, independent of inflammation, by bacterial pathogens.
Though the interactions between infection, immunity, and coagulation are becoming more clear, it remains to be determined if activation of coagulation is a benefit or a detriment to the host. That is, did the coagulation system evolve a way to detect pathogens to in an effort to augment immunity, or did the pathogen evolve a strategy to activate clotting to drive pathogenesis?
When contemplating this question, it is important to note that the vertebrate coagulation system we recognize today has its origins in the innate immune response of invertebrates.8 This early innate immunity focused on the “coagulation” of hemolymph to trap invasive pathogens and to limit bacterial dissemination. Interestingly, the key cells involved in coagulation of hemolymph are hemocytes, a primordial hybrid between neutrophils and platelets, further attesting to the immune-based roots of the vertebrate hemostatic system. Even today, mammalian platelets retain key immune functions including pathogen detection, cytokine production, and the ability to regulate the activation of other immune cells. Studies examining coagulation in the host response to bacterial infection have identified both proresolution and pathogenic roles. Activation of thrombin generates molecules that regulate the host antimicrobial response and support the formation of local thrombi that limit bacterial dissemination.9 In contrast, generation of fibrin contributes to the development of bacterial biofilms, sheltering the pathogen from the immune system and leading to the persistence of infection.10 Additionally, pathogen-induced coagulation results in microvascular occlusion, tissue damage, and subsequent organ failure,1,5 exacerbating the pathogenesis of the infection. Whether coagulation is “good” or “bad” in a given patient is most probably dependent on the specific situation. Localized, limited thrombin and fibrin generation is likely to support the host immune response and to enhance pathogen clearance, whereas uncontrolled, systemic coagulation will result in elevated tissue pathogenesis and worsen patient outcomes.
This multifaceted interaction and interplay between pathogen and the host clotting response highlights the difficulties in treating infection-associated coagulopathies. In any given infection, multiple redundant and overlapping pathways are activated leading to the generation of intravascular thrombi, tissue damage, and organ failure.1,5 Therapies that target a single pathway involved in infection-induced coagulation often fail to improve patient outcomes. Moreover, therapies targeting the terminal generation of thrombin fail to differentiate between coagulopathy and hemostasis, often leading to bleeding events and again resulting in poor patient outcomes. Understanding these multiple interactions between pathogen, inflammation, and coagulation is critical to the development of effective therapeutic strategies for the treatment of infection-associated DICs. Ultimately, a successful therapy should be able to uncouple infection-induced clotting from normal hemostasis, protecting the patient from DIC while preserving the ability to respond appropriately to surgery or traumatic injury. As our understanding of the processes involved improves, so does our ability to treat these patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal