TO THE EDITOR:
Inherited bone marrow failure syndromes (IBMFSs), characterized by bone marrow failure and developmental anomalies, are among a group of rare cancer predisposition syndromes. As illustrated by Fanconi anemia (FA),1 the unique biology of this IBMFS has provided important insights into drivers of malignancy. Diamond-Blackfan anemia (DBA), one of the IBMFSs, is associated with red cell aplasia and congenital anomalies. The majority of cases with DBA result from dominant, loss-of-function mutations/deletions in ∼20 genes encoding either a small or large subunit-associated ribosomal protein (RP) (reviewed in Vlachos et al2 ). We previously described and quantified the cancer risk in DBA with a cumulative incidence of solid tumors (STs) and acute myeloid leukemia (AML) of ∼20% by 46 years of age3 using the Diamond Blackfan Anemia Registry (DBAR) of North America.4,5 As of July 2016, the DBAR consisted of 702 patients, accrued through physician or self-referral. We now report longitudinal data describing a further increase in the incidence of colon cancer and osteogenic sarcoma.
The DBAR of North America (United States, Canada, and Mexico), established in 1991 and registered at www.clinicaltrials.gov (#NCT00106015), is the largest, international DBA patient cohort with prospective follow-up.4,5 Written informed consent as per the Declaration of Helsinki was obtained from all study participants. The current analysis includes an additional 6 years with 94 additional patients and 2918 patient-years than in the previous report. We classify cancer type and age of onset from 702 patients enrolled from November 1991 through July 2016. A competing risks approach was used to estimate cause-specific hazard functions and cumulative incidences for the first adverse event (hematopoietic stem cell transplantation [HSCT], death from nonmalignant causes, AML, or ST) as previously described.3 Results were compared with the expected number of malignancies (observed/expected [O/E] ratio) derived from the Surveillance, Epidemiology and End Results Program (SEER 9) (https://seer.cancer.gov/data/), with adjustment for age, sex, and birth cohort. Pathology reports and medical records were obtained to verify the neoplastic diagnoses in 30% of cases. Tumors not included in SEER were excluded from this analysis and myelodysplastic syndrome (MDS) was analyzed separately. The DBAR is voluntary and is not actively searching for those patients with cancer specifically but enrolls all comers at any age who want to be enrolled. Thus, there may be biased enrollment of older individuals, some with cancer prior to the time of enrollment, as well as a bias toward concern about a perceived increased cancer risk by a parent or patient. In total, there were 8 patients with cancer and one with MDS/AML diagnosed prior to their enrollment (described in Table 1). Of note, a certain bias would be created if enrollment was restricted to only those patients without a cancer diagnosis prior to enrollment in the DBAR.
Cancer and MDS in patients with DBA
| Cancer diagnosis . | No. of patients . | O/E ratio (95% CI) . | Mutated gene . | Age at first cancer diagnosis (y), median (range) . |
|---|---|---|---|---|
| All cancer | 28 | 4.8 (3.2-6.9) | — | 35 (11-70) |
| Gastrointestinal cancer | ||||
| Colon carcinoma | 7 | 44.7 (18.0-92.1) | RPS19 (2; 5 unknown) | 41 (28-51)* |
| Gastroesophageal cancer | 1 | 28.2 (0.7-157.0) | RPS17 | 28 |
| Esophageal cancer | 1 | 64.6 (1.6-359.7) | RPL5 | 69† |
| Sarcoma | ||||
| Osteogenic sarcoma | 4 | 42.4 (11.6-108.7) | All unknown | 18 (11-34) |
| Soft tissue sarcoma | 1 | 6.6 (0.2-36.9) | Unknown | 30 |
| Genitourinary cancer | ||||
| Testicular cancer | 1 | 4.0 (0.1-22.2) | RPL35A | 42 |
| Uterine cancer | 1 | 0 (0-24.1) | RPS19 | 64 |
| Cervical cancer | 1 | 7.1 (0.2-39.3) | RPS19 | 27 |
| Squamous cell carcinoma (vaginal) | 1 | 172.4 (4.4-960.3) | RPL11 | 41 |
| Skin cancer | ||||
| Melanoma | 1 | 2.0 (0.1-11.0) | RPL5 | 50‡ |
| Squamous cell carcinoma (oral) | 1 | 9.1 (0.2-50.7) | RPL11 | 59 |
| Other cancer | ||||
| Breast cancer | 2 | 2.1 (0.3-7.6) | RPS19 (1; 1 unknown) | 34, 43§ |
| Lung cancer | 1 | 9.4 (1.1-33.8) | RPS19 | 49 |
| Choroid meningioma of the lung | 1 | Included in lung cancer above | RPS19 | 21 |
| Hematologic cancer | ||||
| Non-Hodgkin lymphoma | 1 | 3.3 (0.1-19.0) | RPL5 | 41 |
| AML | 3 | 28.8 (5.9-84.0) | RPL35A (2; 1 unknown) | 44 (15-46) |
| MDS | ||||
| MDS | 8 | 352.1 (152.0-693.8) | RPL35A(2), GATA1 (1) (5 unknown) | 26 (2-53) |
| Skin cancer excluded from analysis | ||||
| Basal cell carcinoma | 3 | Not available | All unknown | 54 (29-54) |
| Squamous cell carcinoma | 3 | Not available | RPL11 (1; 2 unknown) | 54 (50-54) |
| Posttransplant cancer excluded from analysis | ||||
| Colorectal carcinoma | 2 | 346.9 (42.0-1253.0) | Both unknown | 19, 29 |
| Osteosarcoma | 1 | 257.8 (6.5-1436.2) | Unknown | 4 |
| Lung cancer | 1 | 468.3 (11.9-2609.4) | RPL11|| | 43 |
| Wilms tumor | 1 | 228.6 (5.8-1273.4) | RPL35A¶ | 8 |
| Cancer diagnosis . | No. of patients . | O/E ratio (95% CI) . | Mutated gene . | Age at first cancer diagnosis (y), median (range) . |
|---|---|---|---|---|
| All cancer | 28 | 4.8 (3.2-6.9) | — | 35 (11-70) |
| Gastrointestinal cancer | ||||
| Colon carcinoma | 7 | 44.7 (18.0-92.1) | RPS19 (2; 5 unknown) | 41 (28-51)* |
| Gastroesophageal cancer | 1 | 28.2 (0.7-157.0) | RPS17 | 28 |
| Esophageal cancer | 1 | 64.6 (1.6-359.7) | RPL5 | 69† |
| Sarcoma | ||||
| Osteogenic sarcoma | 4 | 42.4 (11.6-108.7) | All unknown | 18 (11-34) |
| Soft tissue sarcoma | 1 | 6.6 (0.2-36.9) | Unknown | 30 |
| Genitourinary cancer | ||||
| Testicular cancer | 1 | 4.0 (0.1-22.2) | RPL35A | 42 |
| Uterine cancer | 1 | 0 (0-24.1) | RPS19 | 64 |
| Cervical cancer | 1 | 7.1 (0.2-39.3) | RPS19 | 27 |
| Squamous cell carcinoma (vaginal) | 1 | 172.4 (4.4-960.3) | RPL11 | 41 |
| Skin cancer | ||||
| Melanoma | 1 | 2.0 (0.1-11.0) | RPL5 | 50‡ |
| Squamous cell carcinoma (oral) | 1 | 9.1 (0.2-50.7) | RPL11 | 59 |
| Other cancer | ||||
| Breast cancer | 2 | 2.1 (0.3-7.6) | RPS19 (1; 1 unknown) | 34, 43§ |
| Lung cancer | 1 | 9.4 (1.1-33.8) | RPS19 | 49 |
| Choroid meningioma of the lung | 1 | Included in lung cancer above | RPS19 | 21 |
| Hematologic cancer | ||||
| Non-Hodgkin lymphoma | 1 | 3.3 (0.1-19.0) | RPL5 | 41 |
| AML | 3 | 28.8 (5.9-84.0) | RPL35A (2; 1 unknown) | 44 (15-46) |
| MDS | ||||
| MDS | 8 | 352.1 (152.0-693.8) | RPL35A(2), GATA1 (1) (5 unknown) | 26 (2-53) |
| Skin cancer excluded from analysis | ||||
| Basal cell carcinoma | 3 | Not available | All unknown | 54 (29-54) |
| Squamous cell carcinoma | 3 | Not available | RPL11 (1; 2 unknown) | 54 (50-54) |
| Posttransplant cancer excluded from analysis | ||||
| Colorectal carcinoma | 2 | 346.9 (42.0-1253.0) | Both unknown | 19, 29 |
| Osteosarcoma | 1 | 257.8 (6.5-1436.2) | Unknown | 4 |
| Lung cancer | 1 | 468.3 (11.9-2609.4) | RPL11|| | 43 |
| Wilms tumor | 1 | 228.6 (5.8-1273.4) | RPL35A¶ | 8 |
Bold rows are statistically significant (P < .05). Eight patients had a cancer diagnosis prior to enrollment in the DBAR (choroid meningioma, age 21 years; colon cancer, age 43 years; colon cancer, age 34 y; osteosarcoma, age 14 years; testicular cancer, age 41 years; soft tissue sarcoma, age 30 years; uterine cancer, age 64 years; and breast and colon cancer, ages 43 and 49 years in the same patient), and 1 patient had MDS/AML at age 43 years.
RPS (L), ribosomal protein small (large) subunit associated.
One of the genetically unknown patients had breast cancer.
Patient previously had melanoma.
Patient later had esophageal cancer.
Patient later had colon cancer.
Also heavy smoker.
Gene in unrelated duplicated area (8q24.1) is a putative Wilms tumor–associated proto-oncogene (nephroblastoma overexpressed [NOV]).
The total of 702 enrolled patients contributed 12 376 person-years of follow-up. The male to female ratio was 1:1.02. There were 39 cancers; 5 in post-HSCT patients (Table 1). Fourteen of the 34 cancers in patients without HSCT were verified by pathology/physician reports. The median age at the first cancer (AML and ST, excluding nonmelanoma skin cancers) was 35 years (range, 11-70 years). Three evaluable AML cases and 25 STs were reported in 26 patients without a prior history of HSCT (Table 1).
Some malignancies were excluded from our analyses. Three basal cell carcinomas and 3 squamous cell carcinomas of the skin (median age, 54 years for each, younger than population-incidence) were excluded because basal cell carcinomas and squamous cell carcinomas are not included in the SEER database, leading to 28 statistically evaluable cancers in 26 patients. One hundred patients had received an HSCT. Five post-HSCT malignancies were also excluded from analysis. Three cancers were consistent with those observed in patients without a history of HSCT: 2 colorectal carcinomas at 15 years and 22 months post-HSCT and 1 osteogenic sarcoma 3 years post-HSCT at 29, 19, and 4 years of age, respectively. The others, lung cancer at age 43 years (3 years after the first HSCT and 8 months after the second HSCT) and Wilms tumor at age 8 years (18 months post-HSCT) may not be DBA related (Table 1).
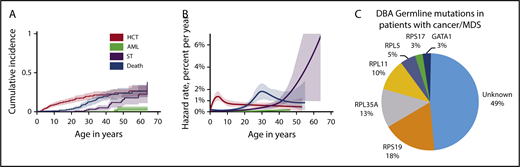
The cumulative incidence of the evaluable cancers was 13.7% by age 45 years (Figure 1A). The cause-specific hazard rates of AML slowly increased beginning around age 40 years, and the rate of STs rapidly increased beginning around age 30 years (Figure 1B). The relative risk of all cancers was significantly increased 4.8-fold compared with the general population. The O/E ratio for colon cancer was 45 and for osteogenic sarcoma 42. Of note, the O/E for all sites in the group of 28 cancers in 26 patients is 4.8 (95% confidence interval [CI], 3.2-6.9). In the patients who underwent HSCT, 3 patients had DBA-related cancers. Of these, the O/E for colorectal cancer in 2 patients and osteosarcoma in 1 patient are 346.9 (95% CI, 42.0-1253.0) and 257.8 (95% CI, 6.5-1436.2), respectively. Although the numbers are small, the CIs do not (or only barely) overlap with the overall O/E for all sites, suggesting that the cancer risk may be higher in the transplanted group. Cancer was not associated with a particular DBA genotype (Figure 1C).
Cumulative incidence, annual hazard rate of competing adverse events by age, and RP germline mutations in patients with cancer. (A) By age 30 years, no patients had developed AML, 3% had a ST, 20% had undergone a HSCT, and 13% had died. By age 45 years, 2% had developed AML, 12% had a ST, 24% had undergone a HSCT, and 23% had died of complications of stem cell transplantation (n = 25), iron overload (n = 18), infection/sepsis (n = 12), gastrointestinal cancer (n = 7), AML (n = 3), pulmonary embolism (n = 3), stroke (n = 2), or aplastic anemia (n = 2). The overall cumulative incidence of cancer (excluding MDS) was 13.7% by age 45 years. (B) Cause-specific hazard rates were as follows: HSCT or severe bone marrow failure occurred at a peak rate of just over 1% per year; deaths from complications of DBA began rising at age 20 years, peaking at almost 2% at 30 years of age; the rate of AML slowly increased beginning at age 40 years; and the rate of STs rapidly increased beginning at age 30 years. (C) DBA germline mutations in patients with cancer/MDS. Twenty of 39 patients with cancer/MDS had an RP gene mutation identified. The relative distribution of DBA in those with cancer genotyped was similar to that reported in the general DBA population with the most common genotypes represented. Due to the availability of only limited genetic testing in earlier patients who had died prior to mutation testing or could not undergo additional complete genetic testing, there were 49% with an unknown genotype. The general DBA population in the DBAR was genotyped (n = 214) as follows: RPS19 (45%), RPL5 (13%), RPS26 (11%), RPL11 (7%), RPL35A (7%), and RPS17 (5%). The significance, if any, of the absence of germline mutations in RPS26 in the cancer cohort is unknown at this time.
Cumulative incidence, annual hazard rate of competing adverse events by age, and RP germline mutations in patients with cancer. (A) By age 30 years, no patients had developed AML, 3% had a ST, 20% had undergone a HSCT, and 13% had died. By age 45 years, 2% had developed AML, 12% had a ST, 24% had undergone a HSCT, and 23% had died of complications of stem cell transplantation (n = 25), iron overload (n = 18), infection/sepsis (n = 12), gastrointestinal cancer (n = 7), AML (n = 3), pulmonary embolism (n = 3), stroke (n = 2), or aplastic anemia (n = 2). The overall cumulative incidence of cancer (excluding MDS) was 13.7% by age 45 years. (B) Cause-specific hazard rates were as follows: HSCT or severe bone marrow failure occurred at a peak rate of just over 1% per year; deaths from complications of DBA began rising at age 20 years, peaking at almost 2% at 30 years of age; the rate of AML slowly increased beginning at age 40 years; and the rate of STs rapidly increased beginning at age 30 years. (C) DBA germline mutations in patients with cancer/MDS. Twenty of 39 patients with cancer/MDS had an RP gene mutation identified. The relative distribution of DBA in those with cancer genotyped was similar to that reported in the general DBA population with the most common genotypes represented. Due to the availability of only limited genetic testing in earlier patients who had died prior to mutation testing or could not undergo additional complete genetic testing, there were 49% with an unknown genotype. The general DBA population in the DBAR was genotyped (n = 214) as follows: RPS19 (45%), RPL5 (13%), RPS26 (11%), RPL11 (7%), RPL35A (7%), and RPS17 (5%). The significance, if any, of the absence of germline mutations in RPS26 in the cancer cohort is unknown at this time.
Eight patients were reported to develop MDS. The actuarial risk of MDS in the absence of any other competing risks was 50% by age 30 years. One patient had MDS and subsequent AML and was tabulated in both categories. Two patients had >1 cancer diagnosis, including 1 patient with breast cancer at age 43 years, colon cancer at age 49 years, and MDS at age 51 years. There are too few patients with DBA in this report to speculate about a predisposition to multiple cancers, unlike patients with Fanconi anemia and dyskeratosis congenita, where multiple cancers are often seen.6
The risk of dying from nonmalignant complications of DBA remains high in patients older than 25 years, and the cumulative incidence of death is 25% by age 50 years (Figure 1A).
Our current analysis with longer follow-up provides additional evidence for an association of DBA with colon cancer and osteogenic sarcoma at a young age, consistent with a hereditary cancer predisposition syndrome.7 Six more years of follow-up also provides stronger evidence that the hazard of STs continues to rise after age 40 years. The appearance of MDS at an early age suggests that the risk of AML may increase as the cohort matures. Elevated incidence of MDS/AML is a common feature in all IBMFSs.6
The IBMFSs FA, dyskeratosis congenita (DC), and DBA have quantifiable ST risks. The most frequent STs in FA are head and neck and gynecologic squamous cell carcinomas. In FA, the O/E ratios for all neoplasms was 19 (95% CI, 12-28). DC also was found to have an elevated O/E ratio of 4.2 (95% CI, 2.8-6.1) for all cancer sites combined.6 The major sites were similar to those for FA. DBA, in our study, had an O/E ratio for all sites of 4.75 and, while significant, is lower than in FA and comparable to DC. Colon cancer and osteogenic sarcoma are not prominent cancers in either FA or DC, suggesting that cancer mechanisms resulting from RP mutations in these tissues, leading to nucleolar stress, differ significantly from those resulting from genomic instability.
A number of recent studies demonstrate somatic mutations in genes encoding RPs in a variety of malignancies, including gastric adenocarcinoma (RPL5 and RPL22) and colorectal cancer (RPL22), as well as the MDS 5q− syndrome (RPS14) (reviewed in Vlachos8 ). Furthermore, RP haploinsufficiency was observed in 43% of a large number of tumor specimens and cancer cell lines.9 The association of RP haploinsufficiency with somatic TP53 mutations suggests that inactivating interdicting mutations of TP53 could result from RP haploinsufficiency.9 RPs have also been demonstrated to be tumor suppressors in zebrafish.10
In conclusion, we expect that the study of the unique cancer predisposition in DBA will reveal important insights into mechanisms of oncogenesis and have specific cancer screening implications for patients with DBA. The efficacy of unbiased cancer surveillance using magnetic resonance imaging has been demonstrated in Li Fraumeni syndrome.7 However, the lower incidence of cancer and different sites in DBA as compared with Li Fraumeni syndrome suggests targeted cancer surveillance strategies may be more appropriate. Investigation of approaches to detect colon cancer in particular (such as colonoscopy), and perhaps osteogenic sarcoma and other malignancies, may be more valuable in DBA.
Acknowledgments
The authors are very grateful to the DBA patients, their parents, and their physicians for contributing data to the DBAR.
This research was supported in part by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (R01 HL 079571-14) (A.V., E.A., and J.M.L.), the Pediatric Cancer Foundation (J.M.L.), the National Cancer Institute, National Institutes of Health (1K07CA216326 - 01A1 and NCI 5 R01 CA211723 02) (R.N.S.), the Patient-Centered Outcomes Research Institute (R.N.S.), and the Intramural Research Program of the National Institutes of Health and the National Cancer Institute (P.S.R. and B.P.A.).
Authorship
Contribution: A.V. and P.S.R. take primary responsibility for this paper; A.V., B.P.A., and J.M.L. designed the DBAR; P.S.R. performed the statistical analysis; A.V., E.A., J.K., and J.M.L. contributed to the collection and review of the patient material; E.A. and J.K. transferred files to the DBAR database; and A.V., P.S.R., E.A., K.O., R.N.S., B.P.A., and J.M.L. contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Adrianna Vlachos, The Feinstein Institute for Medical Research, Pediatric Hematology/Oncology and Stem Cell Transplantation, 350 Community Dr, Manhasset, NY 11030; e-mail: avlachos@northwell.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal