In this issue of Blood, Hind and colleagues leverage a custom microscale system to demonstrate that endothelial cells communicate with neutrophils via interleukin-6 (IL-6) and granulocyte-macrophage colony-stimulating factor (GM-CSF) in the presence of live Pseudomonas aeruginosa to enhance neutrophil transmigration.1
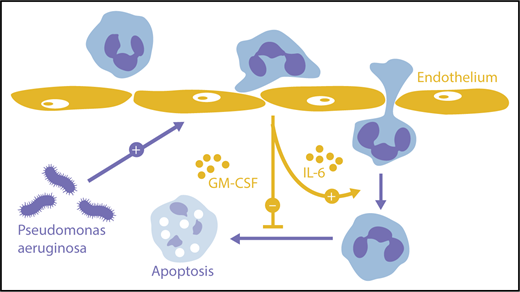
Orchestration of neutrophil transmigration by endothelium. Endothelial cells activated by extraluminal pathogens can provide IL-6 that promotes neutrophil migration deeper into tissues as well as GM-CSF that prolongs neutrophil survival.
Orchestration of neutrophil transmigration by endothelium. Endothelial cells activated by extraluminal pathogens can provide IL-6 that promotes neutrophil migration deeper into tissues as well as GM-CSF that prolongs neutrophil survival.
Neutrophils are a critical first line in the defense against infection but also contribute to pathologic inflammation. Their entry into tissues is therefore carefully regulated by a network of cells, cytokines, chemokines, and adhesion molecules.2 Rolling neutrophils adhere to the endothelial surface, crawl to a location suitable for passage either between or through endothelial cells, and then track along pericytes to penetrate the endothelial basement membrane. Once in the tissues, neutrophils swarm in response to signals provided by each other and by their environment before expiring locally or migrating back into the vasculature to deposit in lung or marrow. In vivo application of 2-photon microscopy, genetic manipulation, and antibody blockade has helped to illuminate these processes in the mouse. However, how can the parts of this network be studied in isolation and in humans?
To address this challenge, some investigators employ microscale assays. For example, customized microfluidics can be used to quantitate neutrophil migration toward chemoattractants and to explore how neutrophils swarm to microbial signals.3 In the present report, Huttenlocher and her laboratory were interested in the role of the endothelium and so devised their own microscale approach in the form of a synthetic blood vessel. Working with the biotechnology group of Beebe, they cast a polymerizable extracellular matrix around a narrow cylindrical mold. The resulting lumen was coated with fibronectin and then cultured human endothelial cells, and fluorescently labeled human peripheral blood neutrophils were observed by video microscopy as they migrated toward P aeruginosa in the extracellular matrix. In this way, the authors could study 3 isolated components of this process: the neutrophil, the endothelium, and the stimulus.
What they found was interesting. The presence of an interposed endothelium enabled neutrophils to penetrate much deeper into the extracellular matrix than neutrophils, leaving an uncoated lumen. This enhancement reflected 2 separate mechanisms. First, endothelium-derived GM-CSF enabled neutrophils to live longer, forestalling apoptosis for up to 24 hours. Second, endothelial IL-6 promoted extended migration, even without any independent effect on survival. Both mediators were elicited from endothelium by the invading microbes, in the case of IL-6 potentially together with neutrophils for maximum cytokine production. Endothelial cells responding to environmental triggers thus promote immune defense by optimizing the neutrophil response to nearby pathogens (see figure, orange arrows).
The role of IL-6 is particularly intriguing. IL-6 plays a key role in multiple inflammatory diseases, and 2 therapies targeting the IL-6 pathway are already approved: tocilizumab, a monoclonal antibody targeting the IL-6 receptor, and tofacitinib, a JAK inhibitor that attenuates signaling by IL-6 as well as other mediators. IL-6 blockade is highly effective in diseases wherein neutrophils appear to play a pivotal role, including rheumatoid arthritis and vasculitis.4,5 The present studies raise the possibility that these agents limit inflammation in part by inhibiting the effect of endothelium-derived IL-6 on neutrophils: potentially contributing also to the infections sometimes seen with these agents.6
The involvement of IL-6 is interesting for other reasons as well. Activated neutrophils release soluble IL-6 receptor, enabling IL-6 to activate endothelial cells and other bystanders that otherwise lack the complete IL-6 receptor.7,8 By encouraging neutrophil recruitment via IL-6, endothelial cells could engage a self-activating amplification loop. Of note, IL-6 is not invariably a net promoter of neutrophil recruitment. Indeed, early experiments considered IL-6 an anti-inflammatory cytokine because IL-6–deficient mice exhibited more neutrophils in lipopolysaccharide-exposed lung than wild-type mice, although in other contexts the lack of IL-6 reduces neutrophil accumulation or has no obvious effect.9,10 In murine and human peritoneum, IL-6 has been implicated in a chemokine “switch” that attenuates neutrophil recruitment in favor of monocytes/macrophages during the resolution phase of inflammation.8 Thus, the results of Hind and colleagues should not be understood as establishing a role for IL-6 for neutrophil recruitment under all circumstances.
The range of findings observed with IL-6 deficiency in vivo highlights the importance of keeping in mind what the system of Hinds and colleagues models and what it does not. The synthetic vessel system tests the interaction of human neutrophils, human-derived endothelium, and a genuine human pathogen. Other elements of the transmigratory environment are omitted, including patrolling monocytes, platelets, pericytes, basement membrane, and alternate sources of chemoattractants, such as fibroblasts, macrophages, and perivascular mast cells. Flow-mediated shear stress is lacking, and the experimental lumen (∼300 µm) is much larger than the postcapillary venules (∼10-50 µm) where neutrophils typically egress. This reductionist system thus shows what role individual components, in this case endothelium, have the potential to play, but the extent to which these roles are decisive in vivo remains to be demonstrated.
Before this study, we knew that endothelial-derived signals participated in the recruitment of neutrophils. We did not know what a major part endothelium could play in neutrophil survival and in their ability to exhibit sustained migration through tissue. The findings thus place the endothelium squarely in the center of the inflammatory orchestra, and so merit our close attention.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal