As Chou et al have elegantly shown in this issue of Blood,1 RH genetic diversity in patients with sickle cell disease (SCD) is prevalent, and matching of donor and recipient red blood cell (RBC) antigens at the molecular level for transfusion is feasible. The Rh blood group system is highly complex, with many antigens defined at the serologic level encoded by the RHD and RHCE genes. Genetic exchange between RHD and RHCE is especially common in individuals of African descent, including those with SCD. In fact, ∼90% of studied patients with SCD have at least 1 RH allele that differs from those found in individuals of European descent.2 Because most blood donors in the United States are not of African descent, each RBC transfusion thus exposes recipients with SCD to many “nonself” blood group antigens.
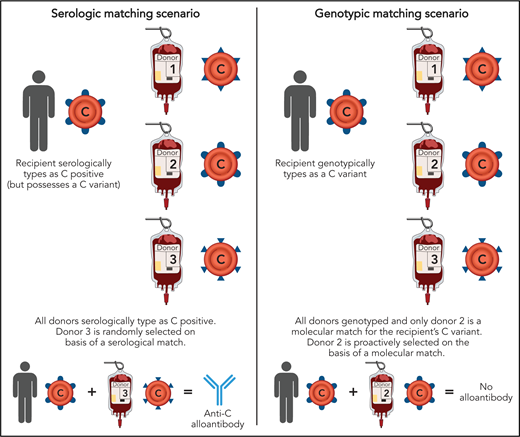
Differences between serologic and genotypic RBC matching. Selection of an RBC donor based on serologic matching may lead to a transfusion recipient with SCD being exposed to a nonself C antigen, for example, potentially resulting in anti-C formation. In contrast, selection of a blood donor based on genotypic matching would mitigate this risk. Professional illustration by Patrick Lane, ScEYEnce Studios.
Differences between serologic and genotypic RBC matching. Selection of an RBC donor based on serologic matching may lead to a transfusion recipient with SCD being exposed to a nonself C antigen, for example, potentially resulting in anti-C formation. In contrast, selection of a blood donor based on genotypic matching would mitigate this risk. Professional illustration by Patrick Lane, ScEYEnce Studios.
Transfusion therapy can be lifesaving for patients living with SCD, treating disease complications such as acute chest syndrome and preventing recurrent ischemic strokes. However, the formation of RBC alloantibodies is a dreaded transfusion complication. Whereas 3% to 5% of the general transfused patient population will become alloimmunized after transfusion, up to 50% of patients with SCD develop RBC alloantibodies.2 Such alloantibodies can be clinically significant in transfusion and pregnancy settings, leading to difficulties in locating compatible RBCs, transfusion reactions, and hemolytic disease of the fetus and newborn. Whereas some transfusion reactions due to non-ABO antibodies can be mild, others, including delayed hemolytic reactions with bystander hemolysis, can be deadly.
Antigens encoded by RHD and RHCE were identified 3 decades ago to be among the most immunogenic in patients with SCD,3 and serologic matching for D, C/c, and E/e between blood donors and recipients has been adopted as a standard of care.4 Although serologic matching decreases alloantibody formation in general, recent studies have reported unexpectedly high rates of antibodies in the RH family despite such matching.2 These high alloimmunization rates could have been dismissed as merely reflecting patients being transfused at multiple hospitals, including hospitals that do not provide phenotype matched RBCs. However, it is now understood that serologic testing, which involves incubating patient/donor RBCs with antisera and looking for agglutination, fails to detect subtle antigenic variants. As Chou et al describe, 21% of patients that phenotyped as C positive actually possessed a “partial” C antigen only revealed by genotyping (with most demonstrating the hybrid RHD*DIIIa-CE(4-7)-D). Without genotyping, these patients would have been routinely transfused with C-positive RBCs and may have developed anti-C despite a prophylactic matching strategy (see figure). Furthermore, 30%, 40%, and 36% of described patients had altered D, c, or e antigens, respectively. Although not intuitive, transfusion recipients with conventional D, C/c, or E/e antigens may also be at risk of forming alloantibodies if exposed to donors expressing variant Rh antigens. Such antibodies, which may have historically been categorized as autoantibodies, are in fact alloantibodies.
The take-home messages of the Chou et al article, which involved RH genotyping 587 blood donors of African descent along with 857 patients with SCD, are that RH genetic diversity is similarly widespread among both groups of individuals, and that provision of genotype matched RBCs is logistically feasible with a donor pool composed primarily of individuals of African descent. No prior study has so thoroughly characterized Rh antigens in patients living with SCD, or determined the large-scale feasibility of RBC provision from a blood donor center perspective. It is important to realize that the Rh variant status of patients with SCD will vary based on region of origin, with patients in the northeastern United States having differences in the distribution of Rh antigenic variants and antibodies formed compared with patients in other regions (eg, France5 ). It is also worth considering that similar genotyping strategies may benefit other highly transfused patient populations at risk of RBC alloimmunization, such as those with myelodysplastic syndrome6 or thalassemia.
One may ask why RBCs from group O, Rh-negative donors cannot a priori be selected for transfusion for individuals with SCD. First, such units are typically collected from donors of European descent, and as such, will express non-Rh antigens that are foreign to recipients of African descent, potentially increasing alloimmunization rates to these antigens. Another consideration is that such units are typically reserved for group O, Rh-negative recipients and for women of childbearing age requiring emergent transfusion. Whereas general transfusion patterns of RBCs have decreased over the past few years, the proportion of group O, Rh-negative RBCs that are distributed has increased by 9%.7 Importantly, over one-third of RBCs transfused to D-positive patients with SCD in 1 study were already from D-negative donors,8 with more selective RBC utilization potentially possible with widespread implementation of RH genotyping on the donor and patient levels.
There are several questions remaining, despite the advancements demonstrated in the work of Chou et al. One question is whether provision of genotype matched RBCs will decrease RBC alloimmunization rates. An additional question is whether other blood donor centers are positioned for such genotype matching, from donor population and logistical perspectives. It must be noted that although genotyping is more accurate than phenotyping,9 the interpretation requires a high level of expertise. Furthermore, this area of personalized medicine is rapidly evolving, with next-generation sequencing able to identify single-nucleotide RH polymorphisms not interrogated by existing DNA arrays.10 Perhaps most important is the “elephant in the room” question: how will genotype (or RBC alloantibody) information be effectively and accurately communicated between different hospitals or blood donor centers, when there is no easily accessible United States–wide database?
In sum, the study by Chou et al in this issue of Blood is a shining example of what can be accomplished when clinicians and researchers with different areas of expertise, including hematology, transfusion medicine, information technology, genomics, and blood donor center operations, collaborate on “Rhesus pieces” and RBC genotype matching. Continued multidisciplinary efforts will allow new frontiers in the care for individuals living with SCD to be developed.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal