Key Points
This comprehensive comparison of the genetic subtypes of hemochromatosis reveals more severe iron overload and disease in non-HFE forms.
Arthropathy is more common in HFE-related hemochromatosis, suggesting that joint disease may not be associated with iron.
Abstract
The clinical progression of HFE-related hereditary hemochromatosis (HH) and its phenotypic variability has been well studied. Less is known about the natural history of non-HFE HH caused by mutations in the HJV, HAMP, or TFR2 genes. The purpose of this study was to compare the phenotypic and clinical presentations of hepcidin-deficient forms of HH. A literature review of all published cases of genetically confirmed HJV, HAMP, and TFR2 HH was performed. Phenotypic and clinical data from a total of 156 patients with non-HFE HH was extracted from 53 publications and compared with data from 984 patients with HFE-p.C282Y homozygous HH from the QIMR Berghofer Hemochromatosis Database. Analyses confirmed that non-HFE forms of HH have an earlier age of onset and a more severe clinical course than HFE HH. HJV and HAMP HH are phenotypically and clinically very similar and have the most severe presentation, with cardiomyopathy and hypogonadism being particularly prevalent findings. TFR2 HH is more intermediate in its age of onset and severity. All clinical outcomes analyzed were more prevalent in the juvenile forms of HH, with the exception of arthritis and arthropathy, which were more commonly seen in HFE HH. This is the first comprehensive analysis comparing the different phenotypic and clinical aspects of the genetic forms of HH, and the results will be valuable for the differential diagnosis and management of these conditions. Importantly, our analyses indicate that factors other than iron overload may be contributing to joint pathology in patients with HFE HH.
Introduction
Hereditary hemochromatosis (HH) is a potentially debilitating genetic disease of systemic iron overload, caused by mutations in genes involved in the regulation of iron homeostasis.1 These mutations lead to increased absorption of iron, resulting in excessive iron deposition in tissues with eventual organ damage and disease. HH results in a clinical spectrum of disease with the potential for cardiac involvement (including cardiomyopathy), hypogonadism, diabetes, skin pigmentation, arthritis, and liver fibrosis.2 Fortunately, early clinical suspicion, pathology testing, diagnosis, and subsequent treatment reverses tissue iron deposition and prevents progression of most of the pathology associated with the disease.
The most common form of HH is caused by homozygosity for the HFE p.C282Y mutation (HFE HH).3,4 Rarely, HFE HH can be caused by other mutations in the HFE gene, including compound heterozygosity for p.C282Y and the more common but less penetrant p.H63D mutation.5,6 Non-HFE forms of HH (non-HFE HH) are caused by mutations in genes involved in iron homeostasis other than HFE. These forms of non-HFE HH are more genetically heterogeneous, with varying patterns of clinical expression.7,8 Similar to HFE HH, these less common genetic conditions can be caused by defects that affect the hepcidin-ferroportin axis, including homozygosity or compound heterozygosity for pathogenic mutations in the genes encoding hemojuvelin (HJV),9 hepcidin (HAMP),10 transferrin receptor 2 (TFR2),11 or heterozygosity for pathogenic mutations in the ferroportin gene (SLC40A1).12,13 HFE HH is predominantly responsible for iron overload cases in populations of European descent. It is of note that non-HFE HH appears to affect both European and non-European populations,14 with case reports from various geographical locations and ethnic groups. Although some forms of non-HFE HH are more severe than HFE HH, they have thus far only been documented in case reports, small case studies,15 and a single meta-analysis of Ferroportin disease.16 No large-scale formal analysis has thus far been performed to compare the phenotypic and clinical features of non-HFE forms of HH.
In the autosomal recessive forms of HH caused by mutations in HFE, HJV, HAMP, or TFR2, hepcidin production by the liver is inadequate to downregulate expression of cell-surface ferroportin, leading to enhanced iron absorption and recycling.1 Hence, these forms of HH have a similar underlying pathophysiology, with phenotypic differences most likely resulting from the degree of impairment in the pathways controlling hepatic hepcidin production. In contrast, mutations in ferroportin can lead to different phenotypic presentations of iron overload, depending on whether the mutation affects the iron transport ability of ferroportin or its sensitivity to hepcidin-mediated downregulation.7
The aims of this study were to compare the phenotypic and clinical disease features of patients with HFE vs those with hepcidin-deficient non-HFE forms of HH to gain a better understanding of the natural history of these iron-associated disorders and aid in their differential diagnosis. This may ultimately provide clinicians and patients with better guidance in the diagnosis, prognostication, and management of iron overload disease both before and after genetic testing. Our analysis has produced the first guide of clinical features matched to serological markers and HH gene mutations, and forms a valuable resource for informing the medical and research community on the clinical comparison of these iron overload disorders.
Methods
Patients
This study was approved by the QIMR Berghofer Human Research Ethics Committee and performed in accordance with the Declaration of Helsinki (1975). For patients derived from the QIMR Berghofer Hemochromatosis Database, informed and written consent was obtained for these studies. Institutional approvals for all other patients were provided in the original publications from which they were derived.
Creation of non-HFE HH database
We conducted a systematic literature review, using PubMed to identify all published cases of genetically confirmed autosomal recessive non-HFE HH caused by mutations in HJV, HAMP, and TFR2 until March 2016, as previously described.17 HH resulting from mutations in SLC40A1 or other genes involved in iron homeostasis outside of the hepcidin-ferroportin axis were not included in this study because of underlying differences in pathophysiology. From the available journal articles, data regarding the genetic, phenotypic, demographic, and clinical outcomes of individual patients were manually extracted and compiled into a database. The extracted data were cross-checked, and the validity of the data were verified by a second investigator. Patients were included if they were homozygous or compound heterozygous for HH-related mutations in the HJV, HAMP, or TFR2 genes. In addition, data from HFE-p.C282Y homozygous individuals from the QIMR Berghofer Hemochromatosis Database was used as a comparison group in the analyses. The following parameters were included in the analyses: genetic cause of HH, sex, age at diagnosis, serum ferritin (SF), transferrin saturation (TS), and the presence of 6 clinical outcomes: cardiac involvement (including cardiomyopathy), hypogonadism, diabetes or abnormalities in blood glucose, skin pigmentation, arthritis or arthropathy, and liver fibrosis or cirrhosis. Publications that did not include the key data of genotype, sex, age at diagnosis, SF, or TS of individual patients were not included in the analyses.
Statistical analyses
Patient data were analyzed using GraphPad Prism version 6 or SPSS version 15.0 with the assistance of an experienced statistician (M.D.C.). Continuous variables were compared between groups using 1-way analysis of variance with Tukey’s multiple comparison test or Kruskal-Wallis with Dunn’s multiple comparison test. Categorical variables were compared between groups using Fisher’s exact test. Logistic regression models were fit to examine how the genetic cause of HH is associated with a clinical feature not adjusting for any variables, adjusting for age group (<25, 25-34, 35-44, 45-54, 55+ years) and sex, adjusting for log SF (linear relation with log odds assumed), and adjusting for age group, sex, and log SF.
Results
Study patients
A total of 156 patients with genetically confirmed autosomal recessive non-HFE HH, with phenotypic and clinical data available, were identified from a total of 53 publications (Table 1). This included 99 HJV (type 2A HH), 11 HAMP (type 2B HH), and 46 TFR2 (type 3 HH) patients. The genotypes associated with these patients and publications from which they were derived are shown in Table 1. From the QIMR Berghofer Hemochromatosis Database, a total of 984 patients with HFE HH resulting from p.C282Y homozygosity were identified for comparison. These HFE HH patients were further subdivided into 558 probands and 426 nonprobands (relatives of probands identified through family screening).
Genotypes of non-HFE HH patients included in this study
| Gene . | Allele 1 . | Allele 2 . | N . | Reference . |
|---|---|---|---|---|
| HJV | p.Leu28SerfsTer24 | p.Leu28SerfsTer24 | 1 | 25 |
| HJV | p.Arg54Ter | p.Arg54Ter | 1 | 26 |
| HJV | p.Gly66Ter | p.Gly66Ter | 1 | 27 |
| HJV | p.Val74TrpfsTer40 | p.Asn269LysfsTer43 | 2 | 15,28 |
| HJV | p.Cys80Arg | p.Leu101Pro | 2 | 29 |
| HJV | p.Cys80Tyr | p.Gly320Val | 1 | 30 |
| HJV | p.Cys80Arg | p.Arg326Ter | 1 | 31 |
| HJV | p.Ser85Pro | p.Ser85Pro | 1 | 28 |
| HJV | p.Cys89Arg | p.Cys89Arg | 2 | 32 |
| HJV | p.Gly99Arg | p.Gly99Arg | 3 | 30 |
| HJV | p.Gly99Val | p.Gly99Val | 1 | 9,33 |
| HJV | p.Gly99Arg | p.Leu101Pro | 1 | 27 |
| HJV | p.Leu101Pro | p.Leu101Pro | 4 | 29 |
| HJV | p.Gln116Ter | p.Gly320Val | 1 | 34 |
| HJV | p.Cys119Phe | p.Cys119Phe | 1 | 35 |
| HJV | p.Arg131PhefsTer111 | p.Arg131PhefsTer111 | 1 | 26 |
| HJV | p.Asp149ThrfsTer97 | p.Asp149ThrfsTer97 | 4 | 15,28 |
| HJV | p.Leu165Ter | p.Leu165Ter | 1 | 36 |
| HJV | p.Ala168Asp | p.Ala168Asp | 1 | 28 |
| HJV | p.Phe170Ser | p.Phe170Ser | 3 | 15,28 |
| HJV | p.Asp172Glu | p.Cys321ValfsTer21 | 1 | 28 |
| HJV | p.Arg176Cys | p.Arg176Cys | 1 | 37 |
| HJV | p.Arg176Cys | p.Gly320Val | 2 | 38,39 |
| HJV | p.Trp191Cys | p.Trp191Cys | 1 | 28 |
| HJV | p.Pro192Leu | p.Pro192Leu | 1 | 30 |
| HJV | p.Leu194Pro | p.Leu194Pro | 1 | 30 |
| HJV | p.Ser205Arg | p.Gly250Val | 2 | 15,28 |
| HJV | p.Ile222Asn | p.Gly320Val | 4 | 9,15,29 |
| HJV | p.Asp249His | p.Asp249His | 1 | 40 |
| HJV | p.Ile281Thr | p.Ile281Thr | 1 | 9 |
| HJV | p.Ile281Thr | p.Cys321Ter | 1 | 41 |
| HJV | p.Arg288Trp | p.Arg288Trp | 3 | 28,42 |
| HJV | p.Gln312Ter | p.Gln312Ter | 5 | 40,43 |
| HJV | p.Gly320Val | p.Gly320Val | 34 | 9,15,20,28,31,35,44-47 |
| HJV | p.Gly320Val | p.Cys321Trp | 1 | 48 |
| HJV | p.Gly320Val | p.Arg326Ter | 1 | 9 |
| HJV | p.Gly320Val | p.Ser328AspfsTer10 | 1 | 35 |
| HJV | p.Ala343ProfsTer24 | p.Ala343ProfsTer24 | 1 | 30 |
| HJV | p.Cys361ValfsTer6 | p.Cys361ValfsTer6 | 1 | 9 |
| HJV | p.Arg385Ter | p.Arg385Ter | 3 | 15,28 |
| HAMP | c.-25G>A | c.-25G>A | 3 | 49,50 |
| HAMP | p.Gly32AspfsTer88 | p.Gly32AspfsTer88 | 2 | 10,33 |
| HAMP | p.Arg42SerfsTer78 | p.Arg42SerfsTer78 | 1 | 30 |
| HAMP | p.Arg56Ter | p.Arg56Ter | 1 | 10,15,21 |
| HAMP | p.Cys70Arg | p.Cys70Arg | 1 | 51,52 |
| HAMP | p.Arg75Ter | p.Arg75Ter | 1 | 53 |
| HAMP | p.Cys78Tyr | p.Cys78Tyr | 2 | 54 |
| TFR2 | p.Glu60Ter | p.Glu60Ter | 6 | 19 |
| TFR2 | p.Glu60Ter | p.Arg105Ter | 1 | 55 |
| TFR2 | p.Leu85_Ala96delinsPro | p.Gly735Ser | 1 | 56 |
| TFR2 | p.Arg105Ter | p.Arg105Ter | 2 | 57 |
| TFR2 | p.Met172Lys | p.Met172Lys | 4 | 19,58,59 |
| TFR2 | c.614+4A>G | c.614+4A>G | 1 | 60 |
| TFR2 | p.Tyr250Ter | p.Tyr250Ter | 8 | 11,61 |
| TFR2 | p.Gln317Ter | p.Gln317Ter | 3 | 62 |
| TFR2 | p.Arg396Ter | c.1538-2A>G | 1 | 63 |
| TFR2 | p.Arg396Ter | p.Gly792Arg | 1 | 64 |
| TFR2 | p.Asn411del | p.Ala444Thr | 1 | 65 |
| TFR2 | p.Asn412Ile | p.Asn412Ile | 1 | 66 |
| TFR2 | p.Gly430Arg | p.Gly430Arg | 1 | 66 |
| TFR2 | p.Gly430Arg | p.Tyr504Cys | 1 | 67 |
| TFR2 | p.Ala444Thr | p.Gly792Arg | 1 | 66 |
| TFR2 | p.Leu490Arg | p.Leu490Arg | 1 | 68 |
| TFR2 | p.Ser556AlafsTer6 | p.Ser556AlafsTer6 | 1 | 68 |
| TFR2 | p.Ala621_Gln624del | p.Ala621_Gln624del | 3 | 69 |
| TFR2 | p.Arg679Pro | p.Arg679Pro | 1 | 66 |
| TFR2 | p.Gln690Pro | p.Gln690Pro | 3 | 70 |
| TFR2 | p.Met705HisfsTer87 | p.Gly792Arg | 1 | 66 |
| TFR2 | c.2137-1G>A | c.2137-1G>A | 2 | 65 |
| TFR2 | p.Arg730Cys | p.Trp781Ter | 1 | 66 |
| Gene . | Allele 1 . | Allele 2 . | N . | Reference . |
|---|---|---|---|---|
| HJV | p.Leu28SerfsTer24 | p.Leu28SerfsTer24 | 1 | 25 |
| HJV | p.Arg54Ter | p.Arg54Ter | 1 | 26 |
| HJV | p.Gly66Ter | p.Gly66Ter | 1 | 27 |
| HJV | p.Val74TrpfsTer40 | p.Asn269LysfsTer43 | 2 | 15,28 |
| HJV | p.Cys80Arg | p.Leu101Pro | 2 | 29 |
| HJV | p.Cys80Tyr | p.Gly320Val | 1 | 30 |
| HJV | p.Cys80Arg | p.Arg326Ter | 1 | 31 |
| HJV | p.Ser85Pro | p.Ser85Pro | 1 | 28 |
| HJV | p.Cys89Arg | p.Cys89Arg | 2 | 32 |
| HJV | p.Gly99Arg | p.Gly99Arg | 3 | 30 |
| HJV | p.Gly99Val | p.Gly99Val | 1 | 9,33 |
| HJV | p.Gly99Arg | p.Leu101Pro | 1 | 27 |
| HJV | p.Leu101Pro | p.Leu101Pro | 4 | 29 |
| HJV | p.Gln116Ter | p.Gly320Val | 1 | 34 |
| HJV | p.Cys119Phe | p.Cys119Phe | 1 | 35 |
| HJV | p.Arg131PhefsTer111 | p.Arg131PhefsTer111 | 1 | 26 |
| HJV | p.Asp149ThrfsTer97 | p.Asp149ThrfsTer97 | 4 | 15,28 |
| HJV | p.Leu165Ter | p.Leu165Ter | 1 | 36 |
| HJV | p.Ala168Asp | p.Ala168Asp | 1 | 28 |
| HJV | p.Phe170Ser | p.Phe170Ser | 3 | 15,28 |
| HJV | p.Asp172Glu | p.Cys321ValfsTer21 | 1 | 28 |
| HJV | p.Arg176Cys | p.Arg176Cys | 1 | 37 |
| HJV | p.Arg176Cys | p.Gly320Val | 2 | 38,39 |
| HJV | p.Trp191Cys | p.Trp191Cys | 1 | 28 |
| HJV | p.Pro192Leu | p.Pro192Leu | 1 | 30 |
| HJV | p.Leu194Pro | p.Leu194Pro | 1 | 30 |
| HJV | p.Ser205Arg | p.Gly250Val | 2 | 15,28 |
| HJV | p.Ile222Asn | p.Gly320Val | 4 | 9,15,29 |
| HJV | p.Asp249His | p.Asp249His | 1 | 40 |
| HJV | p.Ile281Thr | p.Ile281Thr | 1 | 9 |
| HJV | p.Ile281Thr | p.Cys321Ter | 1 | 41 |
| HJV | p.Arg288Trp | p.Arg288Trp | 3 | 28,42 |
| HJV | p.Gln312Ter | p.Gln312Ter | 5 | 40,43 |
| HJV | p.Gly320Val | p.Gly320Val | 34 | 9,15,20,28,31,35,44-47 |
| HJV | p.Gly320Val | p.Cys321Trp | 1 | 48 |
| HJV | p.Gly320Val | p.Arg326Ter | 1 | 9 |
| HJV | p.Gly320Val | p.Ser328AspfsTer10 | 1 | 35 |
| HJV | p.Ala343ProfsTer24 | p.Ala343ProfsTer24 | 1 | 30 |
| HJV | p.Cys361ValfsTer6 | p.Cys361ValfsTer6 | 1 | 9 |
| HJV | p.Arg385Ter | p.Arg385Ter | 3 | 15,28 |
| HAMP | c.-25G>A | c.-25G>A | 3 | 49,50 |
| HAMP | p.Gly32AspfsTer88 | p.Gly32AspfsTer88 | 2 | 10,33 |
| HAMP | p.Arg42SerfsTer78 | p.Arg42SerfsTer78 | 1 | 30 |
| HAMP | p.Arg56Ter | p.Arg56Ter | 1 | 10,15,21 |
| HAMP | p.Cys70Arg | p.Cys70Arg | 1 | 51,52 |
| HAMP | p.Arg75Ter | p.Arg75Ter | 1 | 53 |
| HAMP | p.Cys78Tyr | p.Cys78Tyr | 2 | 54 |
| TFR2 | p.Glu60Ter | p.Glu60Ter | 6 | 19 |
| TFR2 | p.Glu60Ter | p.Arg105Ter | 1 | 55 |
| TFR2 | p.Leu85_Ala96delinsPro | p.Gly735Ser | 1 | 56 |
| TFR2 | p.Arg105Ter | p.Arg105Ter | 2 | 57 |
| TFR2 | p.Met172Lys | p.Met172Lys | 4 | 19,58,59 |
| TFR2 | c.614+4A>G | c.614+4A>G | 1 | 60 |
| TFR2 | p.Tyr250Ter | p.Tyr250Ter | 8 | 11,61 |
| TFR2 | p.Gln317Ter | p.Gln317Ter | 3 | 62 |
| TFR2 | p.Arg396Ter | c.1538-2A>G | 1 | 63 |
| TFR2 | p.Arg396Ter | p.Gly792Arg | 1 | 64 |
| TFR2 | p.Asn411del | p.Ala444Thr | 1 | 65 |
| TFR2 | p.Asn412Ile | p.Asn412Ile | 1 | 66 |
| TFR2 | p.Gly430Arg | p.Gly430Arg | 1 | 66 |
| TFR2 | p.Gly430Arg | p.Tyr504Cys | 1 | 67 |
| TFR2 | p.Ala444Thr | p.Gly792Arg | 1 | 66 |
| TFR2 | p.Leu490Arg | p.Leu490Arg | 1 | 68 |
| TFR2 | p.Ser556AlafsTer6 | p.Ser556AlafsTer6 | 1 | 68 |
| TFR2 | p.Ala621_Gln624del | p.Ala621_Gln624del | 3 | 69 |
| TFR2 | p.Arg679Pro | p.Arg679Pro | 1 | 66 |
| TFR2 | p.Gln690Pro | p.Gln690Pro | 3 | 70 |
| TFR2 | p.Met705HisfsTer87 | p.Gly792Arg | 1 | 66 |
| TFR2 | c.2137-1G>A | c.2137-1G>A | 2 | 65 |
| TFR2 | p.Arg730Cys | p.Trp781Ter | 1 | 66 |
Sex distribution
The sex distribution of patients with non-HFE and HFE HH were compared (supplemental Table 1, available on the Blood Web site). There were similar proportions of males and females in the HJV HH group that did not deviate significantly from the expected ratio of 1:1. There were more males than females in the HAMP and TFR2 groups; however, the ratios did not statistically deviate from the expected 1:1 ratio, possibly because of the low number of patients in these groups. There were significantly more males than females in the HFE proband group (64% vs 35%; binomial test, P < .0001). As expected, the HFE nonproband group, which was identified through family screening, had equal numbers of males and females. These results are consistent with the more severe clinical course seen in males compared with females with HFE HH and the more equal sex distribution that has been observed in patients with juvenile forms of HH.18
Age at diagnosis
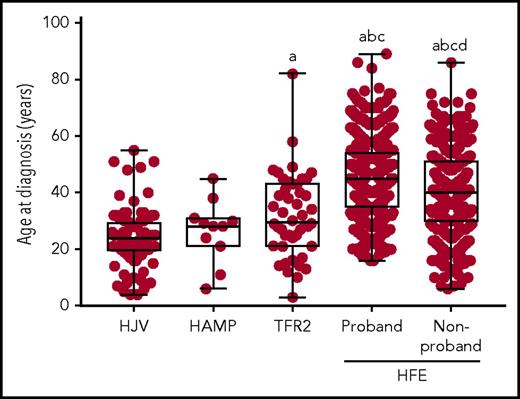
The age at diagnosis was lower for all forms of non-HFE HH studied when compared with HFE HH (Figure 1; supplemental Table 1). Mean age at diagnosis was similar for HJV HH and HAMP HH (24 and 26 years, respectively). Mean age at diagnosis for TFR2 HH (32 years) was significantly higher than for HJV HH, while being intermediate between the juvenile forms of HH and HFE HH (HFE probands: 45 years; HFE nonprobands: 40 years). The spread of age at diagnosis was quite large for all forms of HH (Figure 1), suggesting there is a high level of phenotypic variability among all forms of HH regardless of the genetic cause.
Age at diagnosis of patients with non-HFE and HFE-related HH. The age at diagnosis is shown for patients with HJV-, HAMP-, TFR2-, and HFE-related HH. Patients with HFE HH have been divided into probands and nonprobands. Graphs show individual data points, and box and whisker plots show median value, upper and lower quartiles, and range. Variables were compared using 1-way analysis of variance and Tukey’s multiple comparison test. Statistically significant differences are denoted as (a) compared with HJV, (b) compared with HAMP, (c) compared with TFR2, and (d) compared with HFE proband.
Age at diagnosis of patients with non-HFE and HFE-related HH. The age at diagnosis is shown for patients with HJV-, HAMP-, TFR2-, and HFE-related HH. Patients with HFE HH have been divided into probands and nonprobands. Graphs show individual data points, and box and whisker plots show median value, upper and lower quartiles, and range. Variables were compared using 1-way analysis of variance and Tukey’s multiple comparison test. Statistically significant differences are denoted as (a) compared with HJV, (b) compared with HAMP, (c) compared with TFR2, and (d) compared with HFE proband.
Serum iron indices
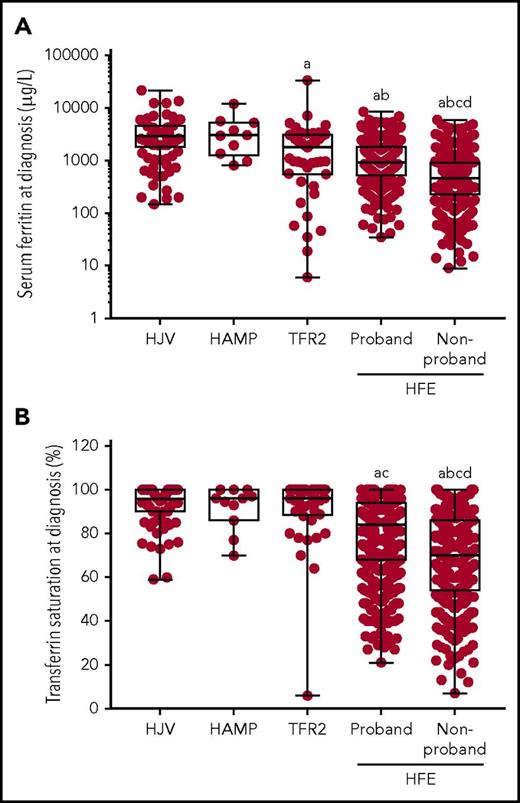
SF concentration and TS were measured in the majority of patients at diagnosis (Figure 2; supplemental Table 1). SF levels, a surrogate marker of storage iron, were greatly elevated in all the non-HFE HH groups (Figure 2A; supplemental Table 1), with similar levels in the HJV and HAMP HH groups (median SF, 2925 and 3050 µg/L, respectively). SF was significantly lower in TFR2 HH (median SF, 1800 µg/L) compared with HJV HH, with a broader spread of values. SF levels were lowest in the HFE HH patients, with the HFE nonprobands (median SF, 463 µg/L) having significantly lower levels compared with the HFE probands (median SF, 937 µg/L).
Serum iron indices at diagnosis. (A) SF (μg/L) and (B) TS (%) at diagnosis are shown for patients with HJV-, HAMP-, TFR2-, and HFE-related HH. Patients with HFE HH have been divided into probands and nonprobands. Graphs show individual data points, and box and whisker plots show the median value, upper, and lower quartiles and range. Variables were compared using the Kruskal-Wallis and Dunn’s multiple comparison tests. Statistically significant differences are denoted as (a) compared with HJV, (b) compared with HAMP, (c) compared with TFR2, and (d) compared with HFE proband.
Serum iron indices at diagnosis. (A) SF (μg/L) and (B) TS (%) at diagnosis are shown for patients with HJV-, HAMP-, TFR2-, and HFE-related HH. Patients with HFE HH have been divided into probands and nonprobands. Graphs show individual data points, and box and whisker plots show the median value, upper, and lower quartiles and range. Variables were compared using the Kruskal-Wallis and Dunn’s multiple comparison tests. Statistically significant differences are denoted as (a) compared with HJV, (b) compared with HAMP, (c) compared with TFR2, and (d) compared with HFE proband.
Similar to SF, TS, a measure of transport iron, was greatly elevated in all non-HFE HH groups, with a median TS of 96% in all 3 groups (Figure 2B; supplemental Table 1). All but 1 of the non-HFE HH patients had TS above 59%, a value well above the normal reference range and indicative of HH. This patient with TFR2 HH also had the lowest SF and was a 43-year-old woman with iron deficiency without anemia, which had been attributed to a history of low dietary iron consumption and blood loss.19 TS was significantly lower in the HFE HH groups and the spread of values was much greater (Figure 2B). The TS was significantly lower in HFE nonprobands (median TS, 70%) compared with the HFE probands (median TS, 84%).
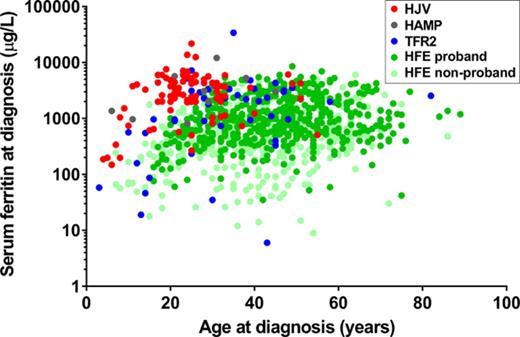
We also analyzed the relationship between serum iron indices and age at diagnosis by plotting age against either SF or TS at diagnosis (supplemental Figure 1). As can be seen in supplemental Figure 1A, there is a wide range of values for age and SF at diagnosis for patients with HH. However, those with HJV or HAMP HH, and to a lesser extent TFR2 HH, cluster toward the upper left of the plot, reflecting the earlier onset and more severe iron loading seen in these forms of HH. A similar observation can be made for age versus TS at diagnosis (supplemental Figure 1B).
Clinical disease features at presentation
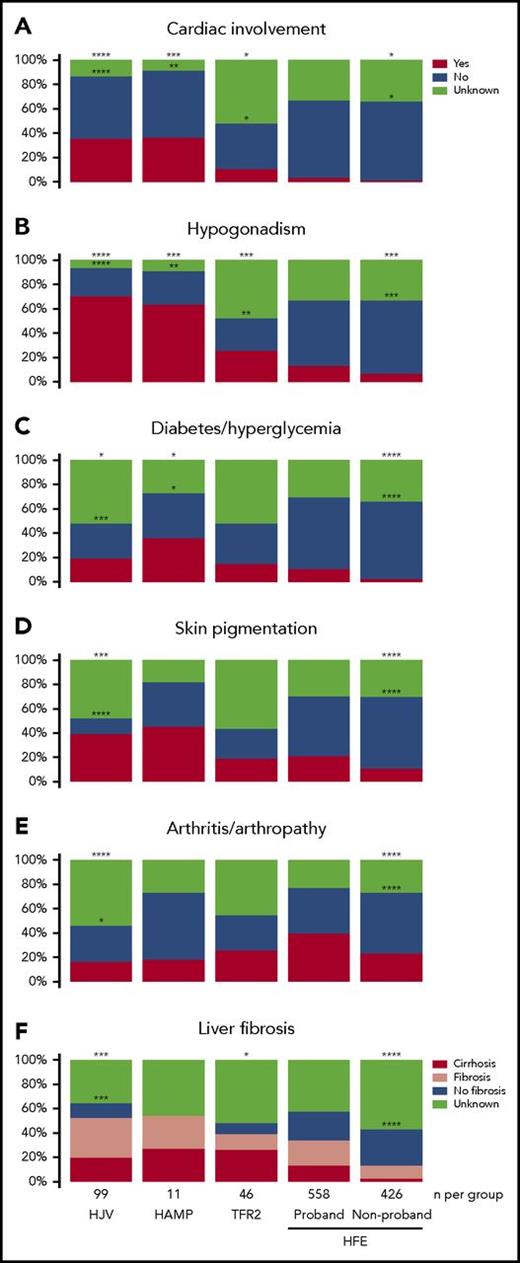
We accounted for the following clinical features in this study: cardiac involvement, hypogonadism, diabetes or hyperglycemia, skin pigmentation, arthritis or arthropathy, and liver fibrosis or cirrhosis (Figure 3; supplemental Table 1). Cardiac involvement and hypogonadism were more prevalent in the non-HFE HH groups when compared with HFE HH (Fisher’s exact test, P < .0001 and P < .0001, respectively). Hypogonadism was also more prevalent in the HJV and HAMP HH groups compared with the TFR2 HH group (75% vs 50%; Fisher’s exact test, P = .023). Cardiac involvement was more prevalent in the HJV and HAMP HH groups compared with the TFR2 HH group (41% vs 23%); however, this did not reach statistical significance, possibly because of the absence of some data in the TFR2 group. These results confirm cardiomyopathy and hypogonadism as hallmark features of the juvenile form of HH.15 Other clinical outcomes such as diabetes/hyperglycemia, skin pigmentation, and liver fibrosis were also more prevalent in the non-HFE compared with HFE groups; however, these only reached statistical significance consistently for the HJV HH group.
Clinical features of patients with non-HFE and HFE-related HH. The presence or absence of the clinical features (A) cardiac involvement, (B) hypogonadism, (C) diabetes/hyperglycemia, (D) skin pigmentation, (E) arthritis/arthropathy, and (F) liver fibrosis were determined in all patients with a genetic diagnosis of HJV-, HAMP-, TFR2-, or HFE-related HH. Patients with HFE HH have been divided into probands and nonprobands. Differences in the prevalence of clinical features between the HFE (proband) group and all other groups were compared using Fisher’s exact test. Statistically significant differences are denoted as ****P < .0001, ***P < .001, **P < .01, and *P < .05. For statistical analyses in (F), liver fibrosis and cirrhosis were combined. For the statistical analyses denoted by asterisks above the boxes, patients with missing data for a particular clinical feature were assumed to not have that clinical feature. For the statistical analyses denoted by asterisks inside the boxes, patients with missing data were excluded.
Clinical features of patients with non-HFE and HFE-related HH. The presence or absence of the clinical features (A) cardiac involvement, (B) hypogonadism, (C) diabetes/hyperglycemia, (D) skin pigmentation, (E) arthritis/arthropathy, and (F) liver fibrosis were determined in all patients with a genetic diagnosis of HJV-, HAMP-, TFR2-, or HFE-related HH. Patients with HFE HH have been divided into probands and nonprobands. Differences in the prevalence of clinical features between the HFE (proband) group and all other groups were compared using Fisher’s exact test. Statistically significant differences are denoted as ****P < .0001, ***P < .001, **P < .01, and *P < .05. For statistical analyses in (F), liver fibrosis and cirrhosis were combined. For the statistical analyses denoted by asterisks above the boxes, patients with missing data for a particular clinical feature were assumed to not have that clinical feature. For the statistical analyses denoted by asterisks inside the boxes, patients with missing data were excluded.
The one exception to the increased prevalence of clinical disease features in non-HFE HH compared with HFE HH was arthritis/arthropathy, a clinical feature that was most prevalent in HFE probands, with statistically lower prevalence in the HJV group (Figure 3). The prevalence of all clinical features was significantly higher in HFE probands compared with HFE nonprobands.
Multivariate analyses
To better understand the relationships among genetics, sex, age, iron indices, and clinical disease features, we performed logistic regression analyses using 4 different models. These analyses examined how the genetic form of HH is associated with a clinical feature, and in the case of HFE HH, whether being a proband or nonproband influences the clinical presentation. For these analyses, we combined the juvenile HH groups, HJV and HAMP, which represent all patients with the classical juvenile form of HH, principally because of the small size of the HAMP group (n = 11) and also because the demographic, phenotypic, and clinical features between these 2 groups were so similar.
The odds ratio (OR) for each of the 6 clinical features are shown for the HJV/HAMP, TFR2, and HFE nonproband groups in comparison with the HFE proband group (Table 2). Regression analysis was performed on clinical features from all patients excluding those with missing data on some of these clinical features (Table 2; supplemental Table 2) or including these patients in the analysis (supplemental Table 3). Similar results were obtained using both approaches. The first regression model did not adjust for any variables (model 1) and showed a statistically significant increase in the OR for the HJV/HAMP group (vs HFE probands) for all 6 clinical features (Table 2), with the OR for cardiac involvement (OR, 11.6) and hypogonadism (OR, 12) being highest (P < .001). The ORs for diabetes/hyperglycemia (OR, 3.8), skin pigmentation (OR, 6), and liver fibrosis (OR, 3.4) were also statistically elevated (P < .001) in the HJV/HAMP group, but the magnitude of change was lower in comparison with cardiac involvement and hypogonadism. The OR for cardiac involvement (OR, 4.9), hypogonadism (OR, 3.9), and liver fibrosis/cirrhosis (OR, 3.1) were statistically higher in the TFR2 group (vs HFE probands), but there were no statistically significant changes in this group for diabetes/hyperglycemia, skin pigmentation, or arthritis/arthropathy.
Logistic regression analyses of clinical features in HH subtypes compared with HFE probands
| Clinical feature and model . | Odds ratio (95% CI) with HFE proband as the reference group . | ||
|---|---|---|---|
| HJV/HAMP . | TFR2 . | HFE nonproband . | |
| Cardiac involvement | |||
| 1 | 11.6 (6.4-21.2)*** | 4.9 (1.6-14.6)** | 0.36 (0.15-0.92)* |
| 2 | 31.3 (10.1--96.9)*** | 7.1 (1.9-26.7)** | 0.67 (0.25-1.8) |
| Hypogonadism | |||
| 1 | 12.0 (7.2-20.2)*** | 3.9 (1.7-9.0)** | 0.46 (0.29-0.72)** |
| 2 | 19.4 (7.8-48.2)*** | 5.2 (1.8-15.3)** | 1.1 (0.62-1.8) |
| Diabetes/hyperglycemia | |||
| 1 | 3.8 (2.1-6.8)*** | 2.4 (0.96-6.2) | 0.23 (0.12-0.44)*** |
| 2 | 8.4 (3.1-23.1)*** | 3.8 (1.2-12.7)* | 0.44 (0.22-0.91)* |
| Skin pigmentation | |||
| 1 | 6.0 (3.3-11.1)*** | 1.8 (0.73-4.4) | 0.43 (0.30-0.63)*** |
| 2 | 12.4 (4.8-32.2)*** | 3.1 (0.93-10.4) | 1.1 (0.68-1.70) |
| Arthritis/arthropathy | |||
| 1 | 0.47 (0.26-0.86)* | 0.85 (0.38-1.9) | 0.45 (0.33-0.61)*** |
| 2 | 1.2 (0.54-2.6) | 1.1 (0.40-2.9) | 0.77 (0.53-1.1) |
| Liver fibrosis/cirrhosis | |||
| 1 | 3.4 (1.7-6.5)*** | 3.1 (1.0-9.4)* | 0.32 (0.22-0.47)*** |
| 2 | 3.0 (0.99-8.8) | 1.9 (0.47-7.9) | 0.55 (0.34-0.88)* |
| Clinical feature and model . | Odds ratio (95% CI) with HFE proband as the reference group . | ||
|---|---|---|---|
| HJV/HAMP . | TFR2 . | HFE nonproband . | |
| Cardiac involvement | |||
| 1 | 11.6 (6.4-21.2)*** | 4.9 (1.6-14.6)** | 0.36 (0.15-0.92)* |
| 2 | 31.3 (10.1--96.9)*** | 7.1 (1.9-26.7)** | 0.67 (0.25-1.8) |
| Hypogonadism | |||
| 1 | 12.0 (7.2-20.2)*** | 3.9 (1.7-9.0)** | 0.46 (0.29-0.72)** |
| 2 | 19.4 (7.8-48.2)*** | 5.2 (1.8-15.3)** | 1.1 (0.62-1.8) |
| Diabetes/hyperglycemia | |||
| 1 | 3.8 (2.1-6.8)*** | 2.4 (0.96-6.2) | 0.23 (0.12-0.44)*** |
| 2 | 8.4 (3.1-23.1)*** | 3.8 (1.2-12.7)* | 0.44 (0.22-0.91)* |
| Skin pigmentation | |||
| 1 | 6.0 (3.3-11.1)*** | 1.8 (0.73-4.4) | 0.43 (0.30-0.63)*** |
| 2 | 12.4 (4.8-32.2)*** | 3.1 (0.93-10.4) | 1.1 (0.68-1.70) |
| Arthritis/arthropathy | |||
| 1 | 0.47 (0.26-0.86)* | 0.85 (0.38-1.9) | 0.45 (0.33-0.61)*** |
| 2 | 1.2 (0.54-2.6) | 1.1 (0.40-2.9) | 0.77 (0.53-1.1) |
| Liver fibrosis/cirrhosis | |||
| 1 | 3.4 (1.7-6.5)*** | 3.1 (1.0-9.4)* | 0.32 (0.22-0.47)*** |
| 2 | 3.0 (0.99-8.8) | 1.9 (0.47-7.9) | 0.55 (0.34-0.88)* |
Logistic regression models were fit to examine how either the genetic form of HH (HFE, HJV/HAMP, or TFR2) and, in the case of HFE HH, whether being a proband or nonproband is associated with a clinical feature, using 2 models: not adjusting for any variables and adjusting for age group (<25, 25-34, 35-44, 45-54, and 55+ years), sex, and log SF. In this analysis, patients with missing data for a particular clinical feature were excluded. Statistically significant differences are denoted as ***P < 0.001, **P < 0.01, *P < 0.05.
Three additional regression models were performed adjusting for age group, sex, and log SF (model 2, Table 2; supplemental Table 3); age group and sex alone (model 3, supplemental Table 2; supplemental Table 3), and log SF alone (model 4, supplemental Table 2 and supplemental Table 3). After adjusting for age group, sex, and log SF (model 2, Table 2), significant associations were still seen in the HJV/HAMP group for cardiac involvement (OR, 31.3), hypogonadism (OR, 19.4), diabetes/hyperglycemia (OR, 8.4), and skin pigmentation (OR, 12.4) when compared with HFE probands. After adjusting for age group, sex, and log SF, significant associations were observed in the TFR2 group for cardiac involvement (OR, 7.1), hypogonadism (OR, 5.2), and diabetes/hyperglycemia (OR, 3.8).
After adjusting for age group and sex alone, the ORs for cardiac involvement, hypogonadism, diabetes/hyperglycemia, skin pigmentation, and liver fibrosis increased in the HJV/HAMP group with more significant associations (model 3, supplemental Table 2). The same was the case for the TFR2 group, although the effect sizes were smaller. Adjusting for log SF decreased the ORs for these 5 clinical features in the HJV/HAMP group, although significant associations were still seen for cardiac involvement, hypogonadism, diabetes/hyperglycemia, and skin pigmentation (model 4, supplemental Table 2). The ORs for cardiac involvement and hypogonadism also remained significant in the TFR2 group after adjusting for log SF (model 4, supplemental Table 2).
In contrast to the other clinical features, the OR for arthritis/arthropathy was significantly lower in the HJV/HAMP group (OR, 0.47) consistent with the lower incidence in this group compared with HFE probands (model 1, Table 2). When adjusted for age group, sex, and log SF (model 2, Table 2) or age group alone (model 3; supplemental Table 2), the association disappeared (OR, 1.2 and 1.7, respectively), suggesting that the lower incidence of arthritis/arthropathy in HJV/HAMP may be a result of the younger age at diagnosis. When adjusted for log SF alone (model 4; supplemental Table 2), the association was stronger (OR, 0.35), suggesting that arthritis in HH may not be solely a result of, or directly related to, iron load.
When comparing the HFE nonproband group with HFE probands, the ORs for all 6 clinical features were significantly lower. After adjusting for age group, sex, and log SF, these associations disappeared for cardiac involvement, hypogonadism, and skin pigmentation. However, ORs were still significantly lower for diabetes/hyperglycemia, liver fibrosis, and to a lesser extent, arthritis/arthropathy after adjusting for these variables.
Discussion
This study is the first comprehensive phenotypic and clinical analysis of patients with hepcidin-deficient forms of HH caused by both HFE and non-HFE genes. The 4 types of HH analyzed (HFE, HJV, HAMP, and TFR2) all result from deficiency of functional hepcidin, the iron-regulatory hormone, with differing phenotypic severities, based on the degree of hepcidin dysregulation. This study analyzed a total of 156 patients with non-HFE HH with a wide variety of genotypes. The 70 different genotypes present across the HJV, HAMP, and TFR2 genes (Table 1) meant that it was difficult to perform any meaningful genotype-phenotype correlations. It is of note that the majority of patients with non-HFE HH (81%) were homozygous for mutations, with the remainder being compound heterozygous. This suggests a high degree of consanguinity among non-HFE HH families, or that families came from isolated populations, an aspect of non-HFE HH genetics that has been alluded to in several published studies.20,21 Whereas the patients with non-HFE HH in this study were derived from global populations, it is of note that a high proportion came from Europe, and in particular Italy (30%) and Greece (10%). Whether this reflects the true distribution of non-HFE forms of HH, or that these forms of HH are recognized, diagnosed, and researched more in these areas, is unclear.
Our analyses confirm that patients with non-HFE HH have an earlier age at diagnosis and more severe iron loading than patients with HFE HH. The 2 subtypes, HJV and HAMP HH, are appropriately classified as “juvenile hemochromatosis,” having an earlier age of onset and the most severe iron loading and clinical course. Clinical presentation for hypogonadism and cardiomyopathy are particularly prevalent in HJV and HAMP HH. TFR2 HH is more intermediate in its onset and severity relative to both the juvenile forms of HH and to HFE HH.
Most clinical features, including cardiac involvement, hypogonadism, diabetes, skin pigmentation, and liver fibrosis, were more prevalent in patients with non-HFE HH compared with HFE HH. Cardiomyopathy and hypogonadism are particularly prevalent clinical findings in HJV and HAMP HH and are strongly associated with these types of HH even after adjusting for age at diagnosis, sex, and SF. The data suggest that in juvenile forms of HH, the earlier and more rapid accumulation of iron has a much more profound effect on the heart and endocrine system, resulting in the higher incidence of cardiomyopathy and hypogonadism seen in patients with HJV- or HAMP-related HH. These results may indicate an increased susceptibility of cardiac tissue and the pituitary to the damaging effects of iron during development. It may also indicate a much earlier deposition of iron in the heart and pituitary in juvenile HH when compared with HFE HH, in which iron deposition in these tissues either occurs much later or not at all. In other organ systems such as the liver, pancreas, and skin, although the effects of iron accumulation can be seen at an earlier age in non-HFE HH, multivariate analyses indicate that the pathology can be mostly attributed to the amount of iron that has accumulated. Previous studies in mouse models of HH indicate that the liver accumulates iron early in all forms of HH but plateaus at different levels, depending on the genetic cause.22 Iron then starts to accumulate more rapidly in the pancreas followed by the heart in juvenile forms of HH. This may be explained as a threshold effect in which, once the liver is iron-loaded to a certain level, iron then accumulates in other organs, including the pancreas, heart, and pituitary, although this remains to be proven. This threshold level in the juvenile forms of HH is likely to be attained at a younger age resulting in the earlier and more damaging effects of iron deposition in the heart and pituitary. In HFE HH, this threshold level may be reached much later in life or may never be achieved in patients with milder phenotypes, leading to the much lower prevalence of cardiomyopathy and hypogonadism in this type of HH.
The relatively high prevalence of arthritis and arthropathy in HFE HH compared with the non-HFE forms of HH is unusual. Although age at diagnosis may partly explain why joint pathologies are more common in HFE HH, adjusting for iron loading as reflected by log SF increases the association of arthritis/arthropathy with HFE HH. This suggests that factors other than body iron stores may be contributing to joint disease in HFE HH. This idea is supported by evidence that venesection treatment does not always relieve joint symptoms in patients with HH and that arthritis can often progress even after iron levels have been normalized.23,24 This is in contrast to other HH-associated pathologies that are normally reversed after venesection treatment.23 There are several possibilities that could explain the higher prevalence of arthritis in HFE HH: HFE may have alternate functions unrelated to systemic iron regulation in joint tissue, or iron may be distributed differently so that it has more damaging effects in the joints of HFE compared with non-HFE patients. Studies that have followed non-HFE patients postdiagnosis and treatment are lacking, so whether they develop arthritis in later life is unclear. The pathophysiology underlying HH-related arthritis has not been well studied, and further research will be required to determine the iron- and noniron-associated factors related to arthritis in HFE HH.
Although this study has analyzed the phenotypic and clinical features of the 4 genetic forms of hepcidin-deficient HH, there are some limitations. First, this study relied on the collection of data from published studies, which did not have standard ways of reporting the data. Second, the non-HFE HH group was limited to 156 patients with adequate data derived from the global literature in comparison with 984 HFE HH patients who were all derived from a single center. This difference likely reflects the rarity of non-HFE forms compared with the HFE form of HH.17 Third, there was a significant amount of missing data; hence, we analyzed the data both with these missing data included and excluded. Finally, as the non-HFE HH data were derived from published studies, there may be some selection bias toward more severely affected cases. However, the same can be said for the comparator group of HFE HH patients, which, similar to most of the cases of non-HFE HH, were derived from referral to a center with research interests in the area of iron-related disorders.
In summary, this analysis provides a reference framework that will be useful to clinicians for the differential diagnosis and management of patients with the various genetic forms of HH. A comprehensive international database for non-HFE HH of clinical and genetic variables will further assist in delineating and defining deficiencies related to iron loading in global populations, allowing for more definitive clinical correlation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported, in part, by Project Grants (APP1031325 and APP1082224) from the National Health and Medical Research Council of Australia (V.N.S.). V.N.S. is the recipient of National Health and Medical Research Council Senior Research Fellowship APP1118888.
Authorship
Contribution: K.S., K.F., and D.F.W. collated the non-HFE HH data; J.L.D., L.E.R., G.A.R., and L.W.P. collated HFE HH data; M.D.C. and D.F.W. analyzed the data and performed statistical analyses; K.S., K.F., M.D.C., V.N.S. and D.F.W. wrote the manuscript; J.L.D., L.E.R., G.A.R., and L.W.P. critically reviewed the manuscript; V.N.S. provided funding; and V.N.S. and D.F.W. designed the studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Daniel F. Wallace, Institute of Health and Biomedical Innovation, 60 Musk Ave, Kelvin Grove, QLD 4059, Australia; e-mail: d5.wallace@qut.edu.au; V. Nathan Subramaniam, Institute of Health and Biomedical Innovation, 60 Musk Ave, Kelvin Grove, QLD 4059, Australia; e-mail: nathan.subramaniam@qut.edu.au.
References
Author notes
V.N.S. and D.F.W. are joint senior and corresponding authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal